Combined effects of chronic nicotine and acute virus exposure on neurotrophin expression in rat lung
- PMID: 19824047
- PMCID: PMC3632456
- DOI: 10.1002/ppul.21099
Combined effects of chronic nicotine and acute virus exposure on neurotrophin expression in rat lung
Abstract
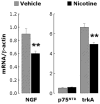
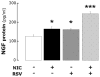
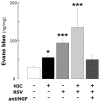
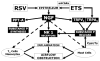
Strong epidemiologic evidence indicates that tobacco smoke influences frequency and severity of respiratory infections. Previously, we have shown that infection with respiratory syncytial virus upregulates expression of neurotrophic factors and receptors in the lungs, but the effect of tobacco exposure on neurotrophins is unknown. Therefore, we first sought to determine the expression of neurotrophic pathways in lungs of rats chronically exposed to nicotine, and then we studied the interactions between pollution and infection by inoculating virus after nicotine exposure. Expression of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor, of their high-affinity tyrosine kinase receptors (trkA and trkB, respectively), and of the low-affinity receptor p75(NTR) was measured in the lungs of nicotine-exposed rats both at the mRNA level by reverse-transcription polymerase chain reaction and at the protein level by enzyme-linked immunoassay. Nicotine increased NGF expression both at the mRNA and protein level and also created a receptor imbalance deriving from increased expression of the pro-inflammatory p75(NTR) receptor without any concomitant change in the high-affinity trkA receptor. Viral infection after chronic nicotine exposure exerted an additive effect on NGF expression, and resulted in exaggerated neurogenic airway inflammation that was abolished by selective inhibition. In conclusion, nicotine levels comparable to those found in smokers are per se able to upregulate the expression of critical neurotrophic molecules in the respiratory tract, and combination of an acute infection following chronic nicotine exposure produces more severe neurotrophic dysregulation and neurogenic-mediated inflammation compared to either infection or nicotine alone.
Figures

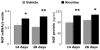

References
-
- Bradley JP, Bacharier LB, Bonfiglio J, Schechtman KB, Strunk R, Storch G, Castro M. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7–e14. - PubMed
-
- Carbonell-Estrany X, Quero J IRIS Study Group. Hospitalization rates for respiratory syncytial virus (RSV) infection in premature infants born during two consecutive seasons. Pediatr Infect Dis J. 2001;20:874–879. - PubMed
-
- Gurkan F, Kiral A, Dagli E, Karakoc F. The effect of passive smoking on the development of respiratory syncytial virus bronchiolitis. Eur J Epidemiol. 2000;16:465–468. - PubMed
-
- McConnochie KM, Roghmann KJ. Parental smoking, presence of older siblings, and family history of asthma increase risk of bronchiolitis. Am J Dis Child. 1986;140:806–812. - PubMed
-
- Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143:S118–S126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

