Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial
- PMID: 19824074
- PMCID: PMC3707633
- DOI: 10.1002/hep.23211
Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial
Abstract
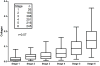
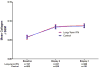
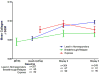
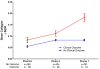
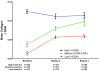
Computer-assisted morphometry can provide precise measurement of hepatic fibrosis on a continuous scale. Previous morphometric studies of large cohorts of patients with treatment refractory chronic hepatitis C have shown a mean increase in fibrosis of 30% to 58% in 1 year. The aim of the present study was to quantify fibrosis progression in biopsy specimens obtained over 1.5 to 5 years from three groups of patients with baseline bridging fibrosis or cirrhosis (Ishak stages 3-6) enrolled in the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. The main group of 346 lead-in nonresponders (viremic after 24 weeks of peginterferon-ribavirin therapy) had a mean fibrosis increase of 61% over pretreatment baseline after 2 years and 80% after 4 years. In contrast, the 78 breakthrough/relapse patients (undetectable serum hepatitis C virus RNA after 24 weeks of peginterferon-ribavirin and receiving antiviral therapy for 48 weeks) showed a mean increase in fibrosis of 48% when biopsied 36 months from pretreatment baseline but no further increase at 60 months. Finally, the 111 express patients with baseline biopsies following unsuccessful peginterferon-ribavirin outside the trial had significantly more baseline fibrosis than the others but an increase of only 21% after 21 months and a slight decrease at 45 months. Maintenance therapy with low-dose peginterferon had no effect on fibrosis changes in any of the groups.
Conclusion: Morphometry demonstrated complex, nonlinear changes in fibrosis over time in this heterogeneous cohort of patients with interferon-refractory chronic hepatitis C.
Figures


References
-
- Imazeki F, Yokosuka O, Fukai K, Saisho H. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology. 2003;38:493–502. - PubMed
-
- Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, Mangia A, Gaeta GB, Persico M, Fagiuoli S, Almasio PL. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–587. - PubMed
-
- Di Marco V, Almasio PL, Ferraro D, Calvaruso V, Alaimo G, Peralta S, Di Stefano R, Craxì A. Peg-interferon alone or combined with ribavirin in HCV cirrhosis with portal hypertension: a randomized controlled trial. J Hepatol. 2007;47:484–491. - PubMed
-
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. - PubMed
-
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, Phillips MJ, Portmann BG, Poulsen H, Scheuer PJ, Schmidt M, Thaler H. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01-DK-9-2325/DK/NIDDK NIH HHS/United States
- 1 UL1 RR024986/RR/NCRR NIH HHS/United States
- 1 UL1 RR025758-01/RR/NCRR NIH HHS/United States
- N01-DK-9-2320/DK/NIDDK NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- M01 RR000065/RR/NCRR NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- N01-DK-9-2322/DK/NIDDK NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- M01RR-00042/RR/NCRR NIH HHS/United States
- 1 UL1 RR 025780-01/RR/NCRR NIH HHS/United States
- N01 DK092324/DK/NIDDK NIH HHS/United States
- N01 DK092321/DK/NIDDK NIH HHS/United States
- M01RR-00065/RR/NCRR NIH HHS/United States
- N01-DK-9-2328/DK/NIDDK NIH HHS/United States
- N01 DK092320/DK/NIDDK NIH HHS/United States
- N01-DK-9-2318/DK/NIDDK NIH HHS/United States
- N01-DK-9-2324/DK/NIDDK NIH HHS/United States
- N01 DK092318/DK/NIDDK NIH HHS/United States
- M01RR-00051/RR/NCRR NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- N01-DK-9-2321/DK/NIDDK NIH HHS/United States
- N01 DK092327/DK/NIDDK NIH HHS/United States
- M01RR-00827/RR/NCRR NIH HHS/United States
- N01-DK-9-2327/DK/NIDDK NIH HHS/United States
- M01RR-01066/RR/NCRR NIH HHS/United States
- 01-DK-9-2319/DK/NIDDK NIH HHS/United States
- 1 UL1 RR024982-01/RR/NCRR NIH HHS/United States
- M01 RR006192/RR/NCRR NIH HHS/United States
- N01 DK092323/DK/NIDDK NIH HHS/United States
- N01 DK092326/DK/NIDDK NIH HHS/United States
- M01 RR000633/RR/NCRR NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- N01-DK-9-2326/DK/NIDDK NIH HHS/United States
- N01 DK092322/DK/NIDDK NIH HHS/United States
- N01-DK-9-2323/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- N01 DK092325/DK/NIDDK NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
- N01 DK092319/DK/NIDDK NIH HHS/United States
- N01 DK092328/DK/NIDDK NIH HHS/United States
- M01RR-00633/RR/NCRR NIH HHS/United States
- M01RR-00043/RR/NCRR NIH HHS/United States
- M01RR-06192/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
