Efficiency of T-cell costimulation by CD80 and CD86 cross-linking correlates with calcium entry
- PMID: 19824921
- PMCID: PMC2807484
- DOI: 10.1111/j.1365-2567.2009.03155.x
Efficiency of T-cell costimulation by CD80 and CD86 cross-linking correlates with calcium entry
Abstract
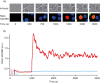
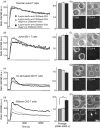
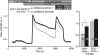
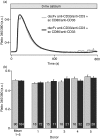
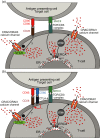
Costimulation is a fundamental principle of T-cell activation. In addition to T-cell receptor engagement, the interaction between CD80 and/or CD86 with CD28 and/or cytotoxic T-lymphocyte antigen 4 (CTLA-4) receptors is required to regulate T-cell activation and tolerance. While the importance of costimulation is clearly established, the exact molecular mechanism is unknown. We demonstrate that T-cell proliferation and the ability of CD8(+) T-effector cells to kill were enhanced slightly by CD80 but dramatically by CD86 costimulation. To further analyse the cellular process of costimulation, we developed a single-cell assay to analyse Ca(2+) signals following costimulation with bi-specific antibodies. We found that this stimulation method worked in every human T-cell that was analysed, making it one of the most efficient T-cell activation methods to date for primary human T cells. The enhanced proliferation and killing by costimulation was paralleled by an increase of Ca(2+) influx following CD86 costimulation and it was dependent on CD28/CTLA-4 expression. The enhanced Ca(2+) influx following CD86 costimulation was abrogated by an antibody that interfered with CD28 function. The differences in Ca(2+) influx between CD80 and CD86 costimulation were not dependent on the depletion of Ca(2+) stores but were eliminated by the application of 10 mum 2-aminoethyldiphenyl borate which has recently been shown to enhance stromal interaction molecule 2 (STIM2)-dependent Ca(2+) entry while reducing STIM1-dependent Ca(2+) entry. Our data indicate that differences in the efficiency of costimulation are linked to differences in Ca(2+) entry.
Figures




References
-
- Friedl P, den Boer AT, Gunzer M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5:532. - PubMed
-
- Jacobelli J, Andres PG, Boisvert J, Krummel MF. New views of the immunological synapse: variations in assembly and function. Curr Opin Immunol. 2004;16:345. - PubMed
-
- Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371. - PubMed
-
- Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2:487. - PubMed
-
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

