Changes in cell migration-related molecules expressed by thymic microenvironment during experimental Plasmodium berghei infection: consequences on thymocyte development
- PMID: 19824923
- PMCID: PMC2814466
- DOI: 10.1111/j.1365-2567.2009.03177.x
Changes in cell migration-related molecules expressed by thymic microenvironment during experimental Plasmodium berghei infection: consequences on thymocyte development
Abstract
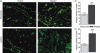
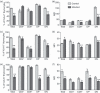
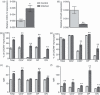
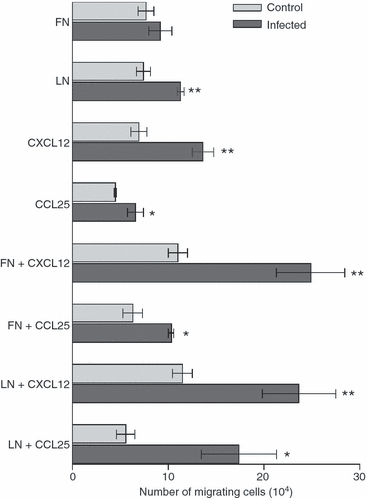
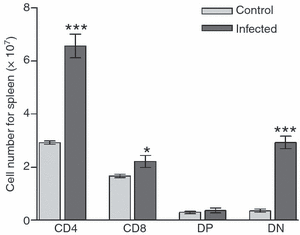
We previously showed alterations in the thymus during experimental infection with Plasmodium berghei. Such alterations comprised histological changes, with loss of cortical-medullary limits, and the intrathymic presence of parasites. As the combination of chemokines, adhesion molecules and extracellular matrix (ECM) is critical to appropriate thymocyte development, we analysed the thymic expression of ECM ligands and receptors, as well as chemokines and their respective receptors during the experimental P. berghei infection. Increased expression of ECM components was observed in thymi from infected mice. In contrast, down-regulated surface expression of fibronectin and laminin receptors was observed in thymocytes from these animals. Moreover, in thymi from infected mice there was increased CXCL12 and CXCR4, and a decreased expression of CCL25 and CCR9. An altered thymocyte migration towards ECM elements and chemokines was seen when the thymi from infected mice were analysed. Evaluation of ex vivo migration patterns of CD4/CD8-defined thymocyte subpopulations revealed that double-negative (DN), and CD4(+) and CD8(+) single-positive (SP) cells from P. berghei-infected mice have higher migratory responses compared with controls. Interestingly, increased numbers of DN and SP subpopulations were found in the spleens of infected mice. Overall, we show that the thymic atrophy observed in P. berghei-infected mice is accompanied by thymic microenvironmental changes that comprise altered expression of thymocyte migration-related molecules of the ECM and chemokine protein families, which in turn can alter the thymocyte migration pattern. These thymic disturbances may have consequences for the control of the immune response against this protozoan.
Figures
Similar articles
-
Effects of Plasmodium berghei on thymus: high levels of apoptosis and premature egress of CD4(+)CD8(+) thymocytes in experimentally infected mice.Immunobiology. 2011 Oct;216(10):1148-54. doi: 10.1016/j.imbio.2011.03.009. Epub 2011 Apr 14. Immunobiology. 2011. PMID: 21601941
-
Impaired migration of NOD mouse thymocytes: a fibronectin receptor-related defect.Eur J Immunol. 2004 Jun;34(6):1578-87. doi: 10.1002/eji.200324765. Eur J Immunol. 2004. PMID: 15162427
-
TNF-α is involved in the abnormal thymocyte migration during experimental Trypanosoma cruzi infection and favors the export of immature cells.PLoS One. 2012;7(3):e34360. doi: 10.1371/journal.pone.0034360. Epub 2012 Mar 26. PLoS One. 2012. PMID: 22461911 Free PMC article.
-
Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix.J Leukoc Biol. 2004 Jun;75(6):951-61. doi: 10.1189/jlb.1003455. Epub 2004 Mar 12. J Leukoc Biol. 2004. PMID: 15020651 Review.
-
Role of extracellular matrix-mediated interactions in thymocyte migration.Dev Immunol. 2000;7(2-4):279-91. doi: 10.1155/2000/60247. Dev Immunol. 2000. PMID: 11097218 Free PMC article. Review.
Cited by
-
Tolerance has its limits: how the thymus copes with infection.Trends Immunol. 2013 Oct;34(10):502-10. doi: 10.1016/j.it.2013.06.004. Epub 2013 Jul 16. Trends Immunol. 2013. PMID: 23871487 Free PMC article. Review.
-
Thymus, undernutrition, and infection: Approaching cellular and molecular interactions.Front Nutr. 2022 Sep 26;9:948488. doi: 10.3389/fnut.2022.948488. eCollection 2022. Front Nutr. 2022. PMID: 36225882 Free PMC article. Review.
-
Infection-Associated Thymic Atrophy.Front Immunol. 2021 May 25;12:652538. doi: 10.3389/fimmu.2021.652538. eCollection 2021. Front Immunol. 2021. PMID: 34113341 Free PMC article. Review.
-
Exacerbation of autoimmune neuro-inflammation in mice cured from blood-stage Plasmodium berghei infection.PLoS One. 2014 Oct 17;9(10):e110739. doi: 10.1371/journal.pone.0110739. eCollection 2014. PLoS One. 2014. PMID: 25329161 Free PMC article.
-
Protein malnutrition promotes dysregulation of molecules involved in T cell migration in the thymus of mice infected with Leishmania infantum.Sci Rep. 2017 Apr 11;7:45991. doi: 10.1038/srep45991. Sci Rep. 2017. PMID: 28397794 Free PMC article.
References
-
- Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. - PubMed
-
- Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–35. - PubMed
-
- Seixas E, Ostler D. Plasmodium chabaudi chabaudi (AS): differential cellular responses to infection in resistant and susceptible mice. Exp Parasitol. 2005;110:394–405. - PubMed
-
- Andrade CF, Gameiro J, Nagib PR, et al. Thymic alterations in Plasmodium berghei-infected mice. Cell Immunol. 2008;253:1–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

