MDA5 and PTPN2, two candidate genes for type 1 diabetes, modify pancreatic beta-cell responses to the viral by-product double-stranded RNA
- PMID: 19825843
- PMCID: PMC2792153
- DOI: 10.1093/hmg/ddp474
MDA5 and PTPN2, two candidate genes for type 1 diabetes, modify pancreatic beta-cell responses to the viral by-product double-stranded RNA
Abstract
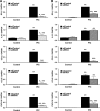
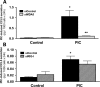
beta-Cell destruction in type 1 diabetes (T1D) is at least in part consequence of a 'dialog' between beta-cells and immune system. This dialog may be affected by the individual's genetic background. We presently evaluated whether modulation of MDA5 and PTPN2, two candidate genes for T1D, affects beta-cell responses to double-stranded RNA (dsRNA), a by-product of viral replication. These genes were selected following comparison between known candidate genes for T1D and genes expressed in pancreatic beta-cells, as identified in previous array analysis. INS-1E cells and primary fluorescence-activated cell sorting-purified rat beta-cells were transfected with small interference RNAs (siRNAs) targeting MDA5 or PTPN2 and subsequently exposed to intracellular synthetic dsRNA (polyinosinic-polycitidilic acid-PIC). Real-time RT-PCR, western blot and viability assays were performed to characterize gene/protein expression and viability. PIC increased MDA5 and PTPN2 mRNA expression, which was inhibited by the specific siRNAs. PIC triggered apoptosis in INS-1E and primary beta-cells and this was augmented by PTPN2 knockdown (KD), although inhibition of MDA5 did not modify PIC-induced apoptosis. In contrast, MDA5 silencing decreased PIC-induced cytokine and chemokine expression, although inhibition of PTPN2 induced minor or no changes in these inflammatory mediators. These findings indicate that changes in MDA5 and PTPN2 expression modify beta-cell responses to dsRNA. MDA5 regulates inflammatory signals, whereas PTPN2 may function as a defence mechanism against pro-apoptotic signals generated by dsRNA. These two candidate genes for T1D may thus modulate beta-cell apoptosis and/or local release of inflammatory mediators in the course of a viral infection by acting, at least in part, at the pancreatic beta-cell level.
Figures



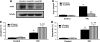

References
-
- Eizirik D.L., Colli M.L., Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009;5:219–226. - PubMed
-
- Harrison L.C., Honeyman M.C., Morahan G., Wentworth J.M., Elkassaby S., Colman P.G., Fourlanos S. Type 1 diabetes: lessons for other autoimmune diseases? J. Autoimmun. 2008;31:306–310. - PubMed
-
- Peng H., Hagopian W. Environmental factors in the development of type 1 diabetes. Rev. Endocr. Metab. Disord. 2006;7:149–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

