The role of callose in guard-cell wall differentiation and stomatal pore formation in the fern Asplenium nidus
- PMID: 19825878
- PMCID: PMC2778399
- DOI: 10.1093/aob/mcp255
The role of callose in guard-cell wall differentiation and stomatal pore formation in the fern Asplenium nidus
Abstract
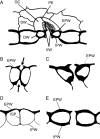
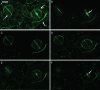
Background and aims: The pattern of callose deposition was followed in developing stomata of the fern Asplenium nidus to investigate the role of this polysaccharide in guard cell (GC) wall differentiation and stomatal pore formation.
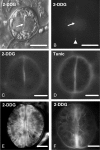
Methods: Callose was localized by aniline blue staining and immunolabelling using an antibody against (1 --> 3)-beta-d-glucan. The study was carried out in stomata of untreated material as well as of material treated with: (1) 2-deoxy-d-glucose (2-DDG) or tunicamycin, which inhibit callose synthesis; (2) coumarin or 2,6-dichlorobenzonitrile (dichlobenil), which block cellulose synthesis; (3) cyclopiazonic acid (CPA), which disturbs cytoplasmic Ca(2+) homeostasis; and (d) cytochalasin B or oryzalin, which disintegrate actin filaments and microtubules, respectively.
Results: In post-cytokinetic stomata significant amounts of callose persisted in the nascent ventral wall. Callose then began degrading from the mid-region of the ventral wall towards its periphery, a process which kept pace with the formation of an 'internal stomatal pore' by local separation of the partner plasmalemmata. In differentiating GCs, callose was consistently localized in the developing cell-wall thickenings. In 2-DDG-, tunicamycin- and CPA-affected stomata, callose deposition and internal stomatal pore formation were inhibited. The affected ventral walls and GC wall thickenings contained membranous elements. Stomata recovering from the above treatments formed a stomatal pore by a mechanism different from that in untreated stomata. After coumarin or dichlobenil treatment, callose was retained in the nascent ventral wall for longer than in control stomata, while internal stomatal pore formation was blocked. Actin filament disintegration inhibited internal stomatal pore formation, without any effect on callose deposition.
Conclusions: In A. nidus stomata the time and pattern of callose deposition and degradation play an essential role in internal stomatal pore formation, and callose participates in deposition of the local GC wall thickenings.
Figures
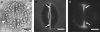







Similar articles
-
Microtubule involvement in the deposition of radial fibrillar callose arrays in stomata of the fern Asplenium nidus L.Cell Motil Cytoskeleton. 2009 Jun;66(6):342-9. doi: 10.1002/cm.20366. Cell Motil Cytoskeleton. 2009. PMID: 19363785
-
Callose implication in stomatal opening and closure in the fern Asplenium nidus.New Phytol. 2010 May;186(3):623-35. doi: 10.1111/j.1469-8137.2010.03206.x. Epub 2010 Mar 8. New Phytol. 2010. PMID: 20298478
-
Microtubule and actin filament organization during stomatal morphogenesis in the fern Asplenium nidus. II. Guard cells.New Phytol. 1999 Feb;141(2):209-223. doi: 10.1046/j.1469-8137.1999.00348.x. New Phytol. 1999. PMID: 33862927
-
A new callose function: involvement in differentiation and function of fern stomatal complexes.Plant Signal Behav. 2010 Nov;5(11):1359-64. doi: 10.4161/psb.5.11.12959. Epub 2010 Nov 1. Plant Signal Behav. 2010. PMID: 21045558 Free PMC article. Review.
-
Callose: a multifunctional (1, 3)-β-D-glucan involved in morphogenesis and function of angiosperm stomata.J Biol Res (Thessalon). 2021 Aug 3;28(1):17. doi: 10.1186/s40709-021-00150-9. J Biol Res (Thessalon). 2021. PMID: 34344461 Free PMC article. Review.
Cited by
-
Polarized and persistent Ca²⁺ plumes define loci for formation of wall ingrowth papillae in transfer cells.J Exp Bot. 2015 Mar;66(5):1179-90. doi: 10.1093/jxb/eru460. Epub 2014 Dec 10. J Exp Bot. 2015. PMID: 25504137 Free PMC article.
-
Identification of Callose Synthases in Stinging Nettle and Analysis of Their Expression in Different Tissues.Int J Mol Sci. 2020 May 28;21(11):3853. doi: 10.3390/ijms21113853. Int J Mol Sci. 2020. PMID: 32481765 Free PMC article.
-
Studies on the Virome of the Entomopathogenic Fungus Beauveria bassiana Reveal Novel dsRNA Elements and Mild Hypervirulence.PLoS Pathog. 2017 Jan 23;13(1):e1006183. doi: 10.1371/journal.ppat.1006183. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28114361 Free PMC article.
-
Cell polarity: compassing cell division and differentiation in plants.Curr Opin Plant Biol. 2018 Oct;45(Pt A):127-135. doi: 10.1016/j.pbi.2018.06.003. Epub 2018 Jun 27. Curr Opin Plant Biol. 2018. PMID: 29957569 Free PMC article. Review.
-
Stomata in Close Contact: The Case of Pancratium maritimum L. (Amaryllidaceae).Plants (Basel). 2022 Dec 5;11(23):3377. doi: 10.3390/plants11233377. Plants (Basel). 2022. PMID: 36501416 Free PMC article.
References
-
- van Amstel TNM, Kengen HMP. Callose deposition in the primary wall of suspension cells and regenerating protoplasts, and its relationship to patterned cellulose synthesis. Canadian Journal of Botany. 1996;74:1040–1049.
-
- Apostolakos P, Galatis B. Probable cytoskeleton involvement in stomatal pore formation in Asplenium nidus L. Protoplasma. 1998;203:48–57.
-
- Apostolakos P, Galatis B. Microtubule and actin filament organization during stomatal morphogenesis in the fern Asplenium nidus. II. Guard cells. New Phytologist. 1999;141:209–223. - PubMed
-
- Apostolakos P, Livanos P, Galatis B. Microtubule involvement in the deposition of radial fibrillar callose arrays in stomata of the fern Asplenium nidus L. Cell Motility and the Cytoskeleton. 2009;66:342–349. - PubMed
-
- Bulone V. In vitro synthesis and analysis of plant (1 → 3)-β-d-glucans and cellulose: a key step towards the characterization of glucan synthases. In: Brown RM Jr, Saxena IM, editors. Cellulose: molecular and structural biology. Heidelberg: Springer; 2007. pp. 123–145.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

