Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor AZD5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours
- PMID: 19825886
- PMCID: PMC2844945
- DOI: 10.1093/annonc/mdp377
Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor AZD5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours
Abstract
Background: AZD5438 is an orally bioavailable inhibitor of cyclin E-cdk2, cyclin A-cdk2 and cyclin B-cdk1 complexes. Three phase I studies assessed the clinical safety, tolerability, pharmacokinetics and pharmacodynamics of AZD5438 when administered in different dosing schedules.
Patients and methods: AZD5438 was administered four times daily, once every 7 days (study 1), for 14 consecutive days followed by 7 days of rest (study 2), or continuously (study 3), to patients with advanced solid tumours. Dose escalation proceeded until the emergence of dose-limiting toxic effects.
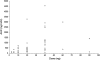
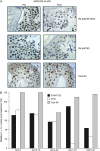
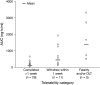
Results: Sixty-four patients were included across the three studies (19, 17 and 28, respectively). Nausea and vomiting were the most common adverse events. When dosed continuously, 40 mg four times daily was considered intolerable, and due to safety issues, all studies were terminated prematurely. Consequently, no intolerable dose was identified during the weekly schedule. Pharmacokinetics demonstrated dose-proportional exposure, high interpatient variability and accumulation after multiple doses. Skin biopsies indicated reduced retinoblastoma protein phosphorylation at cdk2 phospho-sites; other pharmacodynamic assessments did not reveal consistent trends.
Conclusions: AZD5438 was generally well tolerated in a weekly dosing schedule, but not in continuous schedules. The clinical development programme for AZD5438 was discontinued owing to tolerability and exposure data from these studies.
Figures
References
-
- Reed SI. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. - PubMed
-
- Harbour JW, Luo RX, Dei Santi A, et al. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. - PubMed
-
- Pines J. The cell cycle kinases. Semin Cancer Biol. 1994;5:305–313. - PubMed
-
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

