COP9 signalosome interacts ATP-dependently with p97/valosin-containing protein (VCP) and controls the ubiquitination status of proteins bound to p97/VCP
- PMID: 19826004
- PMCID: PMC2787357
- DOI: 10.1074/jbc.M109.037952
COP9 signalosome interacts ATP-dependently with p97/valosin-containing protein (VCP) and controls the ubiquitination status of proteins bound to p97/VCP
Abstract
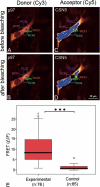
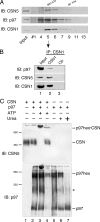
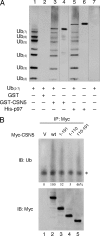
Ubiquitinated proteins can alternatively be delivered directly to the proteasome or via p97/VCP (valosin-containing protein). Whereas the proteasome degrades ubiquitinated proteins, the homohexameric ATPase p97/VCP seems to control the ubiquitination status of recruited substrates. The COP9 signalosome (CSN) is also involved in the ubiquitin/proteasome system (UPS) as exemplified by regulating the neddylation of ubiquitin E3 ligases. Here, we show that p97/VCP colocalizes and directly interacts with subunit 5 of the CSN (CSN5) in vivo and is associated with the entire CSN complex in an ATP-dependent manner. Furthermore, we provide evidence that the CSN and in particular the isopeptidase activity of its subunit CSN5 as well as the associated deubiquitinase USP15 are required for proper processing of polyubiquitinated substrates bound to p97/VCP. Moreover, we show that in addition to NEDD8, CSN5 binds to oligoubiquitin chains in vitro. Therefore, CSN and p97/VCP could form an ATP-dependent complex that resembles the 19 S proteasome regulatory particle and serves as a key mediator between ubiquitination and degradation pathways.
Figures


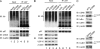
References
-
- Hershko A., Ciechanover A. (1998) Annu. Rev. Biochem. 67, 425–479 - PubMed
-
- Pickart C. M., Cohen R. E. (2004) Nat. Rev. Mol. Cell Biol. 5, 177–187 - PubMed
-
- Wei N., Deng X. W. (2003) Annu. Rev. Cell Dev. Biol. 19, 261–286 - PubMed
-
- Huang X., Hetfeld B. K., Seifert U., Kähne T., Kloetzel P. M., Naumann M., Bech-Otschir D., Dubiel W. (2005) FEBS J 272, 3909–3917 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

