Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions
- PMID: 19826049
- PMCID: PMC4397483
- DOI: 10.1158/0008-5472.CAN-09-0901
Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions
Abstract
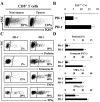
B7-H1 is a recently identified B7 family member that, along with one of its receptors, programmed death-1 (PD-1), has been involved in multiple immunopathologic scenarios. However, the nature of B7-H1 and PD-1 in human hepatocellular carcinoma (HCC) remains poorly defined. We investigated the expression and functional relevance of this pathway in patients with HCC. We showed that B7-H1 expression on Kupffer cells (KC) was increased in tumor tissues compared with surrounding nontumor liver tissues in patients with HCC and this correlated with poorer survival. Coculture of HCC cells with monocytes showed that tumor-associated interleukin-10 contributed to the induction of B7-H1 in the HCC environment. We further observed that the levels of PD-1(+)CD8(+) T cells were higher in tumor tissues than in nontumor tissues. B7-H1(+) KCs and PD-1(+) T cells were colocalized in the HCC stroma. PD-1(+)CD8(+) T cells had decreased proliferative ability and effector function as shown by reduced granule and cytokine expression compared with PD-1(-) T cells. Importantly, blocking KC B7-H1 interaction with PD-1(+)CD8(+) cells using neutralizing antibodies recovered effector T-cell function. Our data indicate that the B7-H1/PD-1 axis contributes to immune suppression in human HCC, with blockade of this pathway carrying important therapeutic implications.
Figures




References
-
- El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–63. - PubMed
-
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. - PubMed
-
- Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93. - PubMed
-
- Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

