Tissue distibution of murine Muc19/smgc gene products
- PMID: 19826070
- PMCID: PMC2803704
- DOI: 10.1369/jhc.2009.954891
Tissue distibution of murine Muc19/smgc gene products
Abstract
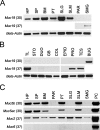
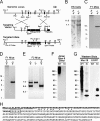
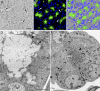
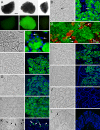
The recently identified gene Muc19/Smgc encodes two diverse splice variants, Smgc (submandibular gland protein C) and Muc19 (mucin 19). Muc19 is a member of the large gel-forming mucin family and is an exocrine product of sublingual mucous salivary glands in mice. SMGC is a transiently expressed secretion product of developing rodent submandibular and sublingual glands. Little is known about the expression of Muc19/Smgc gene products in other murine salivary and non-salivary tissues containing the mucous cell phenotype. Muc19 expression was therefore initially assessed by RT-PCR and immunohistochemistry. As a complementary approach, we developed a knockin mouse model, Muc19-EGFP, in which mice express a fusion protein containing the first 69 residues of Muc19 followed by enhanced green fluorescent protein (EGFP) as a marker of Muc19 expression. Results from both approaches are consistent, with preferential Muc19 expression in salivary major and minor mucous glands as well as submucosal glands of the tracheolarynx and bulbourethral glands. Evidence also indicates that individual mucous cells of minor salivary and bulbourethral glands produce another gel-forming mucin in addition to Muc19. We further find tissue expression of full-length Smgc transcripts, which encode for SMGC, and are restricted to neonatal tracheolarynx and all salivary tissues.
Figures


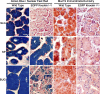
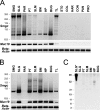
References
-
- Ball WD, Hand AR, Moreira JE, Johnson AO (1988) A secretory protein restricted to type I cells in neonatal rat submandibular glands. Dev Biol 129:464–475 - PubMed
-
- Ball WD, Redman RS (1984) Two independently regulated secretory systems within the acini of the submandibular gland of the perinatal rat. Eur J Cell Biol 33:112–122 - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795 - PubMed
-
- Chen J, Sun M, Hurst LD, Carmichael GG, Rowley JD (2005) Genome-wide analysis of coordinate expression and evolution of human cis-encoded sense-antisense transcripts. Trends Genet 21:326–329 - PubMed
-
- Culp DJ, Latchney LR, Fallon M, Denny PA, Couwenhoven PC, Sally RI, Chuang S (2004) The gene encoding mouse Muc19: cDNA, genomic organization and relationship to Smgc. Physiol Genomics 19:303–318 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

