Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites
- PMID: 19826086
- PMCID: PMC2761242
- DOI: 10.1073/pnas.0907518106
Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites
Abstract
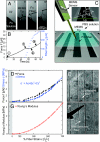
Rather than maximizing toughness, as needed for silk and muscle titin fibers to withstand external impact, the much softer extracellular matrix fibers made from fibronectin (Fn) can be stretched by cell generated forces and display extraordinary extensibility. We show that Fn fibers can be extended more than 8-fold (>700% strain) before 50% of the fibers break. The Young's modulus of single fibers, given by the highly nonlinear slope of the stress-strain curve, changes orders of magnitude, up to MPa. Although many other materials plastically deform before they rupture, evidence is provided that the reversible breakage of force-bearing backbone hydrogen bonds enables the large strain. When tension is released, the nano-sized Fn domains first contract in the crowded environment of fibers within seconds into random coil conformations (molten globule states), before the force-bearing hydrogen bond networks that stabilize the domain's secondary structures are reestablished within minutes (double exponential). The exposure of cryptic binding sites on Fn type III modules increases steeply upon stretching. Thus fiber extension steadily up-regulates fiber rigidity and cryptic epitope exposure, both of which are known to differentially alter cell behavior. Finally, since stress-strain relationships cannot directly be measured in native extracellular matrix (ECM), the stress-strain curves were correlated with stretch-induced alterations of intramolecular fluorescence resonance energy transfer (FRET) obtained from trace amounts of Fn probes (mechanical strain sensors) that can be incorporated into native ECM. Physiological implications of the extraordinary extensibility of Fn fibers and contraction kinetics are discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gosline JM, Guerette PA, Ortlepp CS, Savage KN. The mechanical design of spider silks: From fibroin sequence to mechanical function. J Exp Biol. 1999;202:3295–3303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

