The keratin-related Ouroboros proteins function as immune antigens mediating tail regression in Xenopus metamorphosis
- PMID: 19826093
- PMCID: PMC2775310
- DOI: 10.1073/pnas.0708837106
The keratin-related Ouroboros proteins function as immune antigens mediating tail regression in Xenopus metamorphosis
Abstract
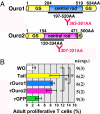
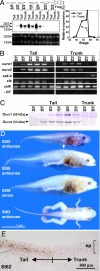
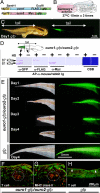
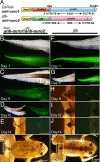
Tail resorption during amphibian metamorphosis has been thought to be controlled mainly by a cell-autonomous mechanism of programmed cell death triggered by thyroid hormone. However, we have proposed a role for the immune response in metamorphosis, based on the finding that syngeneic grafts of tadpole tail skin into adult Xenopus animals are rejected by T cells. To test this, we identified two tail antigen genes called ouro1 and ouro2 that encode keratin-related proteins. Recombinant Ouro1 and Ouro2 proteins generated proliferative responses in vitro in T cells isolated from naive adult Xenopus animals. These genes were expressed specifically in the tail skin at the climax of metamorphosis. Overexpression of ouro1 and ouro2 induced T-cell accumulation and precocious tail degeneration after full differentiation of adult-type T cells when overexpressed in the tail region. When the expression of ouro1 and ouro2 were knocked down, tail skin tissue remained even after metamorphosis was complete. Our findings indicate that Ouro proteins participate in the process of tail regression as immune antigens and highlight the possibility that the acquired immune system contributes not only to self-defense but also to remodeling processes in vertebrate morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ouro proteins are not essential to tail regression during Xenopus tropicalis metamorphosis.Genes Cells. 2016 Mar;21(3):275-86. doi: 10.1111/gtc.12337. Epub 2016 Feb 5. Genes Cells. 2016. PMID: 26847415 Free PMC article.
-
Molecular features of thyroid hormone-regulated skin remodeling in Xenopus laevis during metamorphosis.Dev Growth Differ. 2009 May;51(4):411-27. doi: 10.1111/j.1440-169X.2009.01100.x. Dev Growth Differ. 2009. PMID: 19382937
-
A novel Xenopus laevis larval keratin gene, xlk2: its gene structure and expression during regeneration and metamorphosis of limb and tail.Biochim Biophys Acta. 2006 May;1759(5):216-24. doi: 10.1016/j.bbaexp.2006.05.004. Epub 2006 May 24. Biochim Biophys Acta. 2006. PMID: 16822559
-
[Induction of cell differentiation and programmed cell death in amphibian metamorphosis].Hum Cell. 1997 Sep;10(3):167-74. Hum Cell. 1997. PMID: 9436036 Review. Japanese.
-
The immune system is involved in Xenopus metamorphosis.Front Biosci (Landmark Ed). 2009 Jan 1;14(1):141-9. doi: 10.2741/3235. Front Biosci (Landmark Ed). 2009. PMID: 19273058 Review.
Cited by
-
Ouro proteins are not essential to tail regression during Xenopus tropicalis metamorphosis.Genes Cells. 2016 Mar;21(3):275-86. doi: 10.1111/gtc.12337. Epub 2016 Feb 5. Genes Cells. 2016. PMID: 26847415 Free PMC article.
-
The genus Xenopus as a multispecies model for evolutionary and comparative immunobiology of the 21st century.Dev Comp Immunol. 2011 Sep;35(9):916-23. doi: 10.1016/j.dci.2011.01.014. Epub 2011 Jan 28. Dev Comp Immunol. 2011. PMID: 21277325 Free PMC article. Review.
-
An archetype and scaling of developmental tissue dynamics across species.Nat Commun. 2023 Dec 11;14(1):8199. doi: 10.1038/s41467-023-43902-y. Nat Commun. 2023. PMID: 38081837 Free PMC article.
-
Tail Resorption During Metamorphosis in Xenopus Tadpoles.Front Endocrinol (Lausanne). 2019 Mar 14;10:143. doi: 10.3389/fendo.2019.00143. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30923513 Free PMC article. Review.
-
Apoptosis in amphibian organs during metamorphosis.Apoptosis. 2010 Mar;15(3):350-64. doi: 10.1007/s10495-009-0422-y. Apoptosis. 2010. PMID: 20238476 Free PMC article. Review.
References
-
- Izutsu Y, Kaiho M, Yoshizato K. Different distribution of epidermal basal cells in the anuran larval skin correlates with the skin's region-specific fate at metamorphosis. J Exp Zool. 1993;267:605–615.
-
- Izutsu Y, Tochinai S, Onoe K. Loss of reactivity to pan-cadherin antibody in epidermal cells as a marker for metamorphic alteration of Xenopus skin. Dev Growth Differ. 2000;42:377–383. - PubMed
-
- Allen BM. The results of extirpation of the anterior lobe of the hypophysis and of the thyroid of Rana pipiens larvae. Science. 1916;44:755–758. - PubMed
-
- Das B, et al. Gene expression changes at metamorphosis induced by thyroid hormone in Xenopus laevis tadpoles. Dev Biol. 2006;291:342–355. - PubMed
-
- Buchholz DR, Heimeier RA, Das B, Washington T, Shi YB. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol. 2006;303:576–590. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

