Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group
- PMID: 19826126
- PMCID: PMC2773217
- DOI: 10.1200/JCO.2009.21.8560
Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group
Abstract
Purpose: To determine the risk of ipsilateral breast events in patients with ductal carcinoma in situ (DCIS) treated with local excision without irradiation.
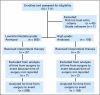
Patients and methods: Patients with either low- or intermediate-grade DCIS measuring 2.5 cm or smaller, or high-grade DCIS measuring 1 cm or smaller who had microscopic margin widths of 3 mm or wider and no residual calcifications on postoperative mammograms were eligible for a prospective trial conducted from 1997 to 2002 by the Eastern Cooperative Oncology Group and North Central Cancer Treatment Group. Patients entered in 2000 and later could take tamoxifen if they wished. Median age at last surgery for the entire population was 60 years (range, 28 to 88 years), and median tumor sizes in the two strata were 6 mm and 5 mm, respectively.
Results: With a median follow-up of 6.2 years, the 5-year rate of ipsilateral breast events in the 565 eligible patients in the low/intermediate grade stratum was 6.1% (95% CI, 4.1% to 8.2%). With a median follow-up of 6.7 years, this incidence for the 105 eligible patients in the high-grade stratum was 15.3% (95% CI, 8.2% to 22.5%).
Conclusion: Rigorously evaluated and selected patients with low- to intermediate-grade DCIS with margins 3 mm or wider had an acceptably low rate of ipsilateral breast events at 5 years after excision without irradiation. Patients with high-grade lesions had a much higher rate, suggesting that excision alone is inadequate treatment. Further follow-up is necessary to document long-term results.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Clinical dilemma of ductal carcinoma in situ.J Clin Oncol. 2009 Nov 10;27(32):5303-5. doi: 10.1200/JCO.2009.24.1489. Epub 2009 Oct 13. J Clin Oncol. 2009. PMID: 19826109 No abstract available.
-
Ductal carcinoma in situ: dilemma or denouement.J Clin Oncol. 2010 May 10;28(14):e218-9; author reply e220. doi: 10.1200/JCO.2009.27.5842. Epub 2010 Apr 5. J Clin Oncol. 2010. PMID: 20368539 No abstract available.
References
-
- Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. - PubMed
-
- Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17. Cancer. 1999;86:429–438. - PubMed
-
- Fisher B, Land S, Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–418. - PubMed
-
- Wapnir I, Dignam J, Julian TB, et al. Long-term outcomes after invasive breast tumor recurrence (IBTR) in women with DCIS in NSABP B-17 and B-24. J Clin Oncol. 2007;25:7s. abstr 520.
-
- Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: Randomised controlled trial. Lancet. 2003;362:95–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA07190/CA/NCI NIH HHS/United States
- CA40057/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA016116/CA/NCI NIH HHS/United States
- CA16116/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- U10 CA007190/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

