Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10
- PMID: 19826132
- PMCID: PMC2773224
- DOI: 10.1200/JCO.2008.20.6490
Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10
Erratum in
- J Clin Oncol. 2010 Mar 10;28(8):1438
Abstract
Purpose: To compare the antitumor efficacy of three different anthracyclines in combination with cytarabine and etoposide in adult patients with newly diagnosed acute myeloid leukemia (AML).
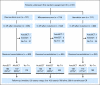
Patients and methods: We randomly assigned 2,157 patients (age range, 15 to 60 years) to receive intensive induction-consolidation chemotherapy containing either daunorubicin, idarubicin, or mitoxantrone. After achieving complete remission (CR), patients were assigned to undergo either allogeneic or autologous stem-cell transplantation (SCT), depending on the availability of a sibling donor.
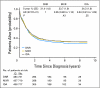
Results: The overall CR rate (69%) was similar in the three groups. Autologous SCT was performed in 37% of cases in the daunorubicin arm versus only 29% and 31% in mitoxantrone and idarubicin, respectively (P < .001). However, the disease-free survival (DFS) and survival from CR were significantly shorter in the daunorubicin arm: the 5-year DFS was 29% versus 37% and 37% in mitoxantrone and idarubicin, respectively. The proportion of patients who underwent allogeneic SCT (22%) was equivalent in the three treatment groups, and the outcome was similar as well. The [corrected] 5-year overall survival rates were 31%, 34%, and 34%, [corrected] respectively.
Conclusion: In adult patients with AML who do not receive an allogeneic SCT, the use of mitoxantrone or idarubicin instead of daunorubicin enhances the long-term efficacy of chemotherapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. - PubMed
-
- Zittoun RA, Mandelli F, Willemze R, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332:217–223. - PubMed
-
- Burnett AK, Goldstone AH, Stevens RM, et al. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: Results of MRC AML 10 trial. UK Medical Research Council Adult and Children's Leukaemia Working Parties. Lancet. 1998;351:700–708. - PubMed
-
- Bishop JF, Lowenthal RM, Joshua D, et al. Etoposide in acute nonlymphocytic leukemia: Australian Leukemia Study Group. Blood. 1990;75:27–32. - PubMed
-
- Bishop JF, Matthews JP, Young GA, et al. Intensified induction chemotherapy with high dose cytarabine and etoposide for acute myeloid leukemia: A review and updated results of the Australian Leukemia Study Group. Leuk Lymphoma. 1998;28:315–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

