Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage
- PMID: 19826415
- PMCID: PMC2795025
- DOI: 10.1038/onc.2009.299
Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage
Abstract
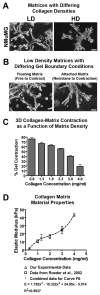
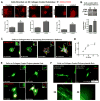
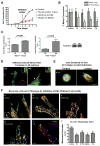
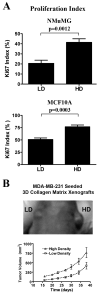
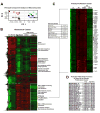
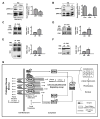
Mammographically dense breast tissue is one of the greatest risk factors for developing breast carcinoma, yet the associated molecular mechanisms remain largely unknown. Importantly, regions of high breast density are associated with increased stromal collagen and epithelial cell content. We set out to determine whether increased collagen-matrix density, in the absence of stromal cells, was sufficient to promote proliferation and invasion characteristic of a malignant phenotype in non-transformed mammary epithelial cells. We demonstrate that increased collagen-matrix density increases matrix stiffness to promote an invasive phenotype. High matrix stiffness resulted in increased formation of activated three-dimensional (3D)-matrix adhesions and a chronically elevated outside-in/inside-out focal adhesion (FA) kinase (FAK)-Rho signaling loop, which was necessary to generate and maintain the invasive phenotype. Moreover, this signaling network resulted in hyperactivation of the Ras-mitogen-activated protein kinase (MAPK) pathway, which promoted growth of mammary epithelial cells in vitro and in vivo and activated a clinically relevant proliferation signature that predicts patient outcome. Hence, the current data provide compelling evidence for the importance of the mechanical features of the microenvironment, and suggest that mechanotransduction in these cells occurs through a FAK-Rho-ERK signaling network with extracellular signal-regulated kinase (ERK) as a bottleneck through which much of the response to mechanical stimuli is regulated. As such, we propose that increased matrix stiffness explains part of the mechanism behind increased epithelial proliferation and cancer risk in human patients with high breast tissue density.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–44. - PubMed
-
- Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Curr Oncol Rep. 2001;3:314–21. - PubMed
-
- Burridge K, Chrzanowska-Wodnicka M. Annu Rev Cell Dev Biol. 1996;12:463–519. - PubMed
-
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Annu Rev Cell Biol. 1988;4:487–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

