A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer
- PMID: 19826418
- PMCID: PMC2778540
- DOI: 10.1038/sj.bjc.6605374
A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer
Abstract
Background: Only a few clinical trials have been conducted in patients with advanced pancreatic cancer after failure of first-line gemcitabine-based chemotherapy. Therefore, there is no current consensus on the treatment of these patients. We conducted a randomised phase II study of the modified FOLFIRI.3 (mFOLFIRI.3; a regimen combining 5-fluorouracil (5-FU), folinic acid, and irinotecan) and modified FOLFOX (mFOLFOX; a regimen combining folinic acid, 5-FU, and oxaliplatin) regimens as second-line treatments in patients with gemcitabine-refractory pancreatic cancer.
Methods: The primary end point was the 6-month overall survival rate. The mFOlFIRI.3 regimen consisted of irinotecan (70 mg m(-2); days 1 and 3), leucovorin (400 mg m(-2); day 1), and 5-FU (2000 mg m(-2); days 1 and 2) every 2 weeks. The mFOLFOX regimen was composed of oxaliplatin (85 mg m(-2); day 1), leucovorin (400 mg m(-2); day 1), and 5-FU (2000 mg m(-2); days 1 and 2) every 2 weeks.
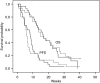
Results: Sixty-one patients were randomised to mFOLFIRI.3 (n=31) or mFOLFOX (n=30) regimen. The six-month survival rates were 27% (95% confidence interval (CI)=13-46%) and 30% (95% CI=15-49%), respectively. The median overall survival periods were 16.6 and 14.9 weeks, respectively. Disease control was achieved in 23% (95% CI=10-42%) and 17% patients (95% CI=6-35%), respectively. The number of patients with at least one grade 3/4 toxicity was identical (11 patients, 38%) in both groups: neutropenia (7 patients under mFOLFIRI.3 regimen vs 6 patients under mFOLFOX regimen), asthaenia (1 vs 4), vomiting (3 in both), diarrhoea (2 vs 0), and mucositis (1 vs 2).
Conclusion: Both mFOLFIRI.3 and mFOLFOX regimens were tolerated with manageable toxicity, offering modest activities as second-line treatments for patients with advanced pancreatic cancer, previously treated with gemcitabine.
Figures
References
-
- Bilimoria K, Bentrem D, Ko C, Ritchey J, Stewart A, Winchester D, Talamonti M (2007) Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer 110: 738–744 - PubMed
-
- Boeck S, Heinemann V (2008) Second-line therapy in gemcitabine-pretreated patients with advanced pancreatic cancer. J Clin Oncol 26: 1178–1179 - PubMed
-
- Brell J, Matin K, Evans T, Volkin R, Kiefer G, Schlesselman J, Dranko S, Rath L, Schmotzer A, Lenzner D, Ramanathan R (2009) Phase II study of docetaxel and gefitinib as second-line therapy in gemcitabine pretreated patients with advanced pancreatic cancer. Oncology 76: 270–274 - PubMed
-
- Burris HR, Moore M, Andersen J, Green M, Rothenberg M, Modiano M, Cripps M, Portenoy R, Storniolo A, Tarassoff P, Nelson R, Dorr F, Stephens C, Von HD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413 - PubMed
-
- Cunningham D, Chau I, Stocken DD (2005) Phase III randomized comparison of gemcitabine (GEM) versus gemcitabine plus capecitabine (GEM-CAP) in patients with advanced pancreatic cancer. Eur J Cancer 3: 3 (abstr)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical