Large-scale parent-child comparison confirms a strong paternal influence on telomere length
- PMID: 19826452
- PMCID: PMC2987222
- DOI: 10.1038/ejhg.2009.178
Large-scale parent-child comparison confirms a strong paternal influence on telomere length
Abstract
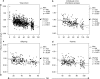
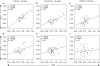
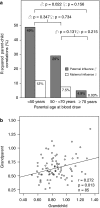
Telomere length is documented to have a hereditary component, and both paternal and X-linked inheritance have been proposed. We investigated blood cell telomere length in 962 individuals with an age range between 0 and 102 years. Telomere length correlations were analyzed between parent-child pairs in different age groups and between grandparent-grandchild pairs. A highly significant correlation between the father's and the child's telomere length was observed (r=0.454, P<0.001), independent of the sex of the offspring (father-son: r=0.465, P<0.001; father-daughter: r=0.484, P<0.001). For mothers, the correlations were weaker (mother-child: r=0.148, P=0.098; mother-son: r=0.080, P=0.561; mother-daughter: r=0.297, P=0.013). A positive telomere length correlation was also observed for grandparent-grandchild pairs (r=0.272, P=0.013). Our findings indicate that fathers contribute significantly stronger to the telomere length of the offspring compared with mothers (P=0.012), but we cannot exclude a maternal influence on the daughter's telomeres. Interestingly, the father-child correlations diminished with increasing age (P=0.022), suggesting that nonheritable factors have an impact on telomere length dynamics during life.
Figures

References
-
- Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. - PubMed
-
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. - PubMed
-
- Iwama H, Ohyashiki K, Ohyashiki JH, et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet. 1998;102:397–402. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

