Design of high-affinity S100-target hybrid proteins
- PMID: 19827097
- PMCID: PMC2821271
- DOI: 10.1002/pro.267
Design of high-affinity S100-target hybrid proteins
Abstract
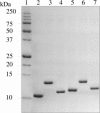
S100B and S100A10 are dimeric, EF-hand proteins. S100B undergoes a calcium-dependent conformational change allowing it to interact with a short contiguous sequence from the actin-capping protein CapZ (TRTK12). S100A10 does not bind calcium but is able to recruit the N-terminus of annexin A2 important for membrane fusion events, and to form larger multiprotein complexes such as that with the cation channel proteins TRPV5/6. In this work, we have designed, expressed, purified, and characterized two S100-target peptide hybrid proteins comprised of S100A10 and S100B linked in tandem to annexin A2 (residues 1-15) and CapZ (TRTK12), respectively. Different protease cleavage sites (tobacco etch virus, PreScission) were incorporated into the linkers of the hybrid proteins. In situ proteolytic cleavage monitored by (1)H-(15)N HSQC spectra showed the linker did not perturb the structures of the S100A10-annexin A2 or S100B-TRTK12 complexes. Furthermore, the analysis of the chemical shift assignments ((1)H, (15)N, and (13)C) showed that residues T102-S108 of annexin A2 formed a well-defined alpha-helix in the S100A10 hybrid while the TRTK12 region was unstructured at the N-terminus with a single turn of alpha-helix from D108-K111 in the S100B hybrid protein. The two S100 hybrid proteins provide a simple yet extremely efficient method for obtaining high yields of intact S100 target peptides. Since cleavage of the S100 hybrid protein is not necessary for structural characterization, this approach may be useful as a scaffold for larger S100 complexes.
Figures

Similar articles
-
The S100A10-annexin A2 complex provides a novel asymmetric platform for membrane repair.J Biol Chem. 2011 Nov 18;286(46):40174-83. doi: 10.1074/jbc.M111.244038. Epub 2011 Sep 26. J Biol Chem. 2011. PMID: 21949189 Free PMC article.
-
Solution NMR structure of S100B bound to the high-affinity target peptide TRTK-12.J Mol Biol. 2002 Dec 13;324(5):1003-14. doi: 10.1016/s0022-2836(02)01152-x. J Mol Biol. 2002. PMID: 12470955
-
The effects of CapZ peptide (TRTK-12) binding to S100B-Ca2+ as examined by NMR and X-ray crystallography.J Mol Biol. 2010 Mar 12;396(5):1227-43. doi: 10.1016/j.jmb.2009.12.057. Epub 2010 Jan 4. J Mol Biol. 2010. PMID: 20053360 Free PMC article.
-
Unique S100 target protein interactions.Gen Physiol Biophys. 2009;28 Spec No Focus:F39-46. Gen Physiol Biophys. 2009. PMID: 20093725 Review.
-
Regulation of CFTR function by annexin A2-S100A10 complex in health and disease.Gen Physiol Biophys. 2009;28 Spec No Focus:F14-9. Gen Physiol Biophys. 2009. PMID: 20093721 Review.
Cited by
-
Structures reveal details of small molecule binding to cardiac troponin.J Mol Cell Cardiol. 2016 Dec;101:134-144. doi: 10.1016/j.yjmcc.2016.10.016. Epub 2016 Nov 5. J Mol Cell Cardiol. 2016. PMID: 27825981 Free PMC article. Review.
-
S100A4 in Cancer Metastasis: Wnt Signaling-Driven Interventions for Metastasis Restriction.Cancers (Basel). 2016 Jun 20;8(6):59. doi: 10.3390/cancers8060059. Cancers (Basel). 2016. PMID: 27331819 Free PMC article. Review.
-
The S100A10-annexin A2 complex provides a novel asymmetric platform for membrane repair.J Biol Chem. 2011 Nov 18;286(46):40174-83. doi: 10.1074/jbc.M111.244038. Epub 2011 Sep 26. J Biol Chem. 2011. PMID: 21949189 Free PMC article.
-
Alteration by p11 of mGluR5 localization regulates depression-like behaviors.Mol Psychiatry. 2015 Dec;20(12):1546-56. doi: 10.1038/mp.2015.132. Epub 2015 Sep 15. Mol Psychiatry. 2015. PMID: 26370144 Free PMC article.
-
Thermodynamic and kinetic analysis of peptides derived from CapZ, NDR, p53, HDM2, and HDM4 binding to human S100B.Biochemistry. 2012 Sep 11;51(36):7189-201. doi: 10.1021/bi300865g. Epub 2012 Aug 29. Biochemistry. 2012. PMID: 22913742 Free PMC article.
References
-
- Deloulme JC, Assard N, Mbele GO, Mangin C, Kuwano R, Baudier J. S100A6 and S100A11 are specific targets of the calcium- and zinc-binding S100B protein in vivo. J Biol Chem. 2000;275:35302–35310. - PubMed
-
- Propper C, Huang X, Roth J, Sorg C, Nacken W. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J Biol Chem. 1999;274:183–188. - PubMed
-
- Wang G, Zhang S, Fernig DG, Martin-Fernandez M, Rudland PS, Barraclough R. Mutually antagonistic actions of S100A4 and S100A1 on normal and metastatic phenotypes. Oncogene. 2005;24:1445–1454. - PubMed
-
- Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

