Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis
- PMID: 19827166
- PMCID: PMC2799538
- DOI: 10.1002/hep.23276
Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis
Abstract
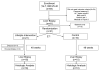
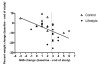
Nonalcoholic steatohepatitis (NASH) is a chronic progressive liver disease that is strongly associated with obesity. Currently, there is no approved therapy for NASH. Weight reduction is typically recommended, but efficacy data are lacking. We performed a randomized controlled trial to examine the effects of lifestyle intervention using a combination of diet, exercise, and behavior modification, with a goal of 7% to 10% weight reduction, on clinical parameters of NASH. The primary outcome measure was the change in NASH histological activity score (NAS) after 48 weeks of intervention. Thirty-one overweight or obese individuals (body mass index [BMI], 25-40 kg/m(2)) with biopsy-proven NASH were randomized in a 2:1 ratio to receive intensive lifestyle intervention (LS) or structured education (control). After 48 weeks of intervention, participants assigned to LS lost an average of 9.3% of their weight versus 0.2% in the control group (P = 0.003). A higher proportion of participants in the LS group had a reduction of NAS of at least 3 points or had posttreatment NAS of 2 or less as compared with the control group (72% versus 30%, P = 0.03). NAS improved significantly in the LS group (from 4.4 to 2.0) in comparison with the control group (from 4.9 to 3.5) (P = 0.05). Percent weight reduction correlated significantly with improvement in NAS (r = 0.497, P = 0.007). Participants who achieved the study weight loss goal (>or=7%), compared with those who lost less than 7%, had significant improvements in steatosis (-1.36 versus -0.41, P < 0.001), lobular inflammation (-0.82 versus -0.24, P = 0.03), ballooning injury (-1.27 versus -0.53, P = 0.03) and NAS (-3.45 versus -1.18, P < 0.001).
Conclusion: Weight reduction achieved through lifestyle intervention leads to improvements in liver histology in NASH.
Figures



References
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. - PubMed
-
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. - PubMed
-
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. - PubMed
-
- McCullough AJ. Thiazolidinediones for nonalcoholic steatohepatitis--promising but not ready for prime time. N Engl J Med. 2006;355:2361–2363. - PubMed
-
- Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
