Chloride channels: often enigmatic, rarely predictable
- PMID: 19827947
- PMCID: PMC2851227
- DOI: 10.1146/annurev-physiol-021909-135811
Chloride channels: often enigmatic, rarely predictable
Abstract
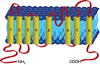
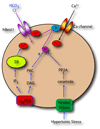
Until recently, anion (Cl(-)) channels have received considerably less attention than cation channels. One reason for this may be that many Cl(-) channels perform functions that might be considered cell-biological, like fluid secretion and cell volume regulation, whereas cation channels have historically been associated with cellular excitability, which typically happens more rapidly. In this review, we discuss the recent explosion of interest in Cl(-) channels, with special emphasis on new and often surprising developments over the past five years. This is exemplified by the findings that more than half of the ClC family members are antiporters, and not channels, as was previously thought, and that bestrophins, previously prime candidates for Ca(2+)-activated Cl(-) channels, have been supplanted by the newly discovered anoctamins and now hold a tenuous position in the Cl(-) channel world.
Figures




References
Literature Cited
-
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinaur Associates Inc.; 1984.
-
- Hogben CA. Physiochemical aspects of hydrochloric acid formation. Am J Dig.Dis. 1959;4:184–193. - PubMed
-
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinaur Associates Inc.; 1992.
Related Resources
-
- Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases. Elsevier; 2009. (in press, expected August, 2009)
-
- Fuller CM, editor. Calcium-activated Chloride Channels. Current Topics in Biomembranes. Volume 53. Academic Press; 2002. 441 pages ISBN-13: 978-0-12-153353-3.
-
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

