A novel inhibitor of Chlamydophila pneumoniae protein kinase D (PknD) inhibits phosphorylation of CdsD and suppresses bacterial replication
- PMID: 19828035
- PMCID: PMC2765968
- DOI: 10.1186/1471-2180-9-218
A novel inhibitor of Chlamydophila pneumoniae protein kinase D (PknD) inhibits phosphorylation of CdsD and suppresses bacterial replication
Abstract
Background: We have shown previously that Chlamydophila pneumoniae contains a dual-specific Ser/Thr protein kinase that phosphorylates CdsD, a structural component of the type III secretion apparatus. To further study the role of PknD in growth and development we sought to identify a PknD inhibitor to determine whether PknD activity is required for replication.
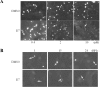
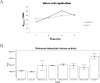
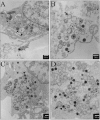
Results: Using an in vitro kinase assay we screened 80 known eukaryotic protein kinase inhibitors for activity against PknD and identified a 3'-pyridyl oxindole compound that inhibited PknD autophosphorylation and phosphorylation of CdsD. The PknD inhibitor significantly retarded the growth rate of C. pneumoniae as evidenced by the presence of very small inclusions with a reduced number of bacteria as seen by electron microscopy. These inclusions contained the normal replicative forms including elementary bodies (EB), intermediate bodies (IB) and reticulate bodies (RB), but lacked persistent bodies (PB), indicating that induction of persistence was not the cause of reduced chlamydial growth. Blind passage of C. pneumoniae grown in the presence of this PknD inhibitor for 72 or 84 hr failed to produce inclusions, suggesting this compound blocks an essential step in the production of infectious chlamydial EB. The compound was not toxic to HeLa cells, did not block activation of the MEK/ERK pathway required for chlamydial invasion and did not block intracellular replication of either Chlamydia trachomatis serovar D or Salmonella enterica sv. Typhimurium suggesting that the inhibitory effect of the compound is specific for C. pneumoniae.
Conclusion: We have identified a 3'-pyridyl oxindole compound that inhibits the in vitro kinase activity of C. pneumoniae PknD and inhibits the growth and production of infectious C. pneumoniae progeny in HeLa cells. Together, these results suggest that PknD may play a key role in the developmental cycle of C. pneumoniae.
Figures



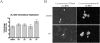

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

