Analysis and modelling of motility of cell populations with MotoCell
- PMID: 19828072
- PMCID: PMC2762061
- DOI: 10.1186/1471-2105-10-S12-S12
Analysis and modelling of motility of cell populations with MotoCell
Abstract
Background: Cell motility plays a central role in development, wound-healing and tumour invasion. Cultures of eukaryotic cells are a complex system where most cells move according to 'random' patterns, but may also be induced to a more coordinate migration by means of specific stimuli, such as the presence of chemical attractants or the introduction of a mechanical stimulus. Various tools have been developed that work by keeping track of the paths followed by specific objects and by performing statistical analysis on the recorded path data. The available tools include desktop applications or macros running within a commercial package, which address specific aspects of the process.
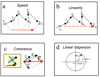
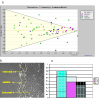
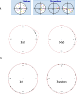
Results: An online application, MotoCell, was developed to evaluate the motility of cell populations maintained in various experimental conditions. Statistical analysis of cell behaviour consists of the evaluation of descriptive parameters such as average speed and angle, directional persistence, path vector length, calculated for the whole population as well as for each cell and for each step of the migration; in this way the behaviour of a whole cell population may be assessed as a whole or as a sum of individual entities. The directional movement of objects may be studied by eliminating the modulo effect in circular statistics analysis, able to evaluate linear dispersion coefficient (R) and angular dispersion (S) values together with average angles. A case study is provided where the system is used to characterize motility of RasV12 transformed NIH3T3 fibroblasts.
Conclusion: Here we describe a comprehensive tool which takes care of all steps in cell motility analysis, including interactive cell tracking, path editing and statistical analysis of cell movement, all within a freely available online service. Although based on a standard web interface, the program is very fast and interactive and is immediately available to a large number of users, while exploiting the web approach in a very effective way. The ability to evaluate the behaviour of single cells allows to draw the attention on specific correlations, such as linearity of movement and deviation from the expected direction. In addition to population statistics, the analysis of single cells allows to group the cells into subpopulations, or even to evaluate the behaviour of each cell with respect to a variable reference, such as the direction of a wound or the position of the closest cell.
Figures





References
-
- Stupack DG. The biology of integrins. Oncology (Williston Park) 2007;21:6–12. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

