Interleukin-2 therapy in patients with HIV infection
- PMID: 19828532
- PMCID: PMC2869083
- DOI: 10.1056/NEJMoa0903175
Interleukin-2 therapy in patients with HIV infection
Abstract
Background: Used in combination with antiretroviral therapy, subcutaneous recombinant interleukin-2 raises CD4+ cell counts more than does antiretroviral therapy alone. The clinical implication of these increases is not known.
Methods: We conducted two trials: the Subcutaneous Recombinant, Human Interleukin-2 in HIV-Infected Patients with Low CD4+ Counts under Active Antiretroviral Therapy (SILCAAT) study and the Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT). In each, patients infected with the human immunodeficiency virus (HIV) who had CD4+ cell counts of either 50 to 299 per cubic millimeter (SILCAAT) or 300 or more per cubic millimeter (ESPRIT) were randomly assigned to receive interleukin-2 plus antiretroviral therapy or antiretroviral therapy alone. The interleukin-2 regimen consisted of cycles of 5 consecutive days each, administered at 8-week intervals. The SILCAAT study involved six cycles and a dose of 4.5 million IU of interleukin-2 twice daily; ESPRIT involved three cycles and a dose of 7.5 million IU twice daily. Additional cycles were recommended to maintain the CD4+ cell count above predefined target levels. The primary end point of both studies was opportunistic disease or death from any cause.
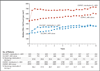
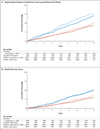
Results: In the SILCAAT study, 1695 patients (849 receiving interleukin-2 plus antiretroviral therapy and 846 receiving antiretroviral therapy alone) who had a median CD4+ cell count of 202 cells per cubic millimeter were enrolled; in ESPRIT, 4111 patients (2071 receiving interleukin-2 plus antiretroviral therapy and 2040 receiving antiretroviral therapy alone) who had a median CD4+ cell count of 457 cells per cubic millimeter were enrolled. Over a median follow-up period of 7 to 8 years, the CD4+ cell count was higher in the interleukin-2 group than in the group receiving antiretroviral therapy alone--by 53 and 159 cells per cubic millimeter, on average, in the SILCAAT study and ESPRIT, respectively. Hazard ratios for opportunistic disease or death from any cause with interleukin-2 plus antiretroviral therapy (vs. antiretroviral therapy alone) were 0.91 (95% confidence interval [CI], 0.70 to 1.18; P=0.47) in the SILCAAT study and 0.94 (95% CI, 0.75 to 1.16; P=0.55) in ESPRIT. The hazard ratios for death from any cause and for grade 4 clinical events were 1.06 (P=0.73) and 1.10 (P=0.35), respectively, in the SILCAAT study and 0.90 (P=0.42) and 1.23 (P=0.003), respectively, in ESPRIT.
Conclusions: Despite a substantial and sustained increase in the CD4+ cell count, as compared with antiretroviral therapy alone, interleukin-2 plus antiretroviral therapy yielded no clinical benefit in either study. (ClinicalTrials.gov numbers, NCT00004978 [ESPRIT] and NCT00013611 [SILCAAT study].)
2009 Massachusetts Medical Society
Figures

Comment in
-
Interleukin-2 therapy in patients with HIV infection.N Engl J Med. 2010 Jan 21;362(3):270-1; author reply 271. doi: 10.1056/NEJMc0910951. N Engl J Med. 2010. PMID: 20089981 No abstract available.
-
Evaluating the clinical benefit of interleukin-2 plus antiretroviral therapy: is raising CD4+ cell counts sufficient?Curr HIV/AIDS Rep. 2010 Feb;7(1):1-3. doi: 10.1007/s11904-009-0032-x. Curr HIV/AIDS Rep. 2010. PMID: 20425051 No abstract available.
References
-
- Kovacs JA, Baseler M, Dewar RJ, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection: a preliminary study. N Engl J Med. 1995;332:567–575. - PubMed
-
- Kovacs JA, Vogel S, Albert JM, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–1356. - PubMed
-
- Davey RT, Jr, Murphy RL, Graziano FM, et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: a randomized controlled trial. JAMA. 2000;284:183–189. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
