Role of nuclear factor Y in stress-induced activation of the herpes simplex virus type 1 ICP0 promoter
- PMID: 19828605
- PMCID: PMC2798407
- DOI: 10.1128/JVI.01377-09
Role of nuclear factor Y in stress-induced activation of the herpes simplex virus type 1 ICP0 promoter
Abstract
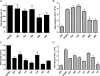
Herpesviruses are characterized by the ability to establish lifelong latent infections and to reactivate periodically, leading to recurrent disease. The herpes simplex virus type 1 (HSV-1) genome is maintained in a quiescent state in sensory neurons during latency, which is characterized by the absence of detectable viral protein synthesis. Cellular factors induced by stress may act directly on promoters within the latent viral genome to induce the transcription of viral genes and trigger reactivation. In order to identify which viral promoters are induced by stress and elucidate the cellular mechanism responsible for the induction, we generated a panel of HSV-1 promoter-luciferase constructs and measured their response to heat shock. Of the promoters tested, those of ICP0 and ICP22 were the most strongly upregulated after heat shock. Microarray analysis of lytically infected cells supported the upregulation of ICP0 and ICP22 promoters by heat shock. Mutagenic analysis of the ICP0 promoter identified two regions necessary for efficient heat-induced promoter activity, both containing predicted nuclear factor Y (NF-Y) sites, at bases -708 and -75 upstream of the transcriptional start site. While gel shift analysis confirmed NF-Y binding to both sites, only the site at -708 was important for efficient heat-induced activity. Reverse transcription-PCR analysis of selected viral transcripts in the presence of dominant-negative NF-Y confirmed the requirement for NF-Y in the induction of the ICP0 but not the ICP22 promoter by heat shock in lytically infected cells. These findings suggest that the immediate-early ICP0 gene may be among the first genes to be induced during the early events in HSV-1 reactivation, that NF-Y is important for this induction, and that other factors induce the ICP22 promoter.
Figures



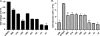



References
-
- Ackland-Berglund, C. E., D. J. Davido, and D. A. Leib. 1995. The roles of the cAMP-response element and TATA box in expression of the herpes simplex virus type 1 latency-associated transcripts. Virology 210:141-151. - PubMed
-
- Ashburner, M., and J. J. Bonner. 1979. The induction of gene activity in Drosophila by heat shock. Cell 17:241-254. - PubMed
-
- Bi, W., L. Wu, F. Coustry, B. de Crombrugghe, and S. N. Maity. 1997. DNA binding specificity of the CCAAT-binding factor CBF/NF-Y. J. Biol. Chem. 272:26562-26572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

