Interactions and oligomerization of hantavirus glycoproteins
- PMID: 19828613
- PMCID: PMC2798430
- DOI: 10.1128/JVI.00481-09
Interactions and oligomerization of hantavirus glycoproteins
Abstract
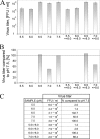
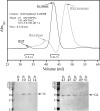
In this report the basis for the structural architecture of the envelope of hantaviruses, family Bunyaviridae, is systematically studied by the interactions of two glycoproteins N and C (Gn and Gc, respectively) and their respective disulfide bridge-mediated homo- and heteromeric oligomerizations. In virion extracts Gn and Gc associated in both homo- and hetero-oligomers which were, at least partially, thiol bridge mediated. Due to strong homo-oligomerization, the hetero-oligomers of Gn and Gc are likely to be mediated by homo-oligomeric subunits. A reversible pH-induced disappearance of a neutralizing epitope in Gc and dissociation of the Gn-Gc complex at pH values below 6.2 provide proteochemical evidence for the fusogenicity of Gc. Incomplete inactivation of virions at acidic pH indicates that additional factors are required for hantavirus fusion, as in the case of pestiviruses of the Flaviviridae. Based on similarities to class II fusion proteins, a structure model was created of hantavirus Gc using the Semliki Forest virus E1 protein as a template. In total, 10 binding regions for Gn were found by peptide scanning, of which five represent homotypic (Gn(I) to Gn(V)) and five represent heterotypic (Gc(I) to Gc(V)) interaction sites that we assign as intra- and interspike connections, respectively. In conclusion, the glycoprotein associations were compiled to a model wherein the surface of hantaviruses is formed of homotetrameric Gn complexes interconnected with Gc homodimers. This organization would create the grid-like surface pattern described earlier for hantaviruses in negatively stained electron microscopy specimens.
Figures







References
-
- Adrian, M., J. Dubochet, J. Lepault, and A. W. McDowall. 1984. Cryo-electron microscopy of viruses. Nature 308:32-36. - PubMed
-
- Arikawa, J., A. L. Schmaljohn, J. M. Dalrymple, and C. S. Schmaljohn. 1989. Characterization of Hantaan virus envelope glycoprotein antigenic determinants defined by monoclonal antibodies. J. Gen. Virol. 70:615-624. - PubMed
-
- Arnold, K., L. Bordoli, J. Kopp, and T. Schwede. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195-201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

