The effects of influenza A virus PB1-F2 protein on polymerase activity are strain specific and do not impact pathogenesis
- PMID: 19828614
- PMCID: PMC2798424
- DOI: 10.1128/JVI.01785-09
The effects of influenza A virus PB1-F2 protein on polymerase activity are strain specific and do not impact pathogenesis
Abstract
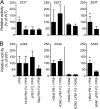
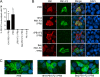
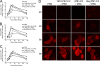
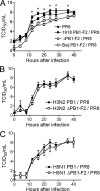
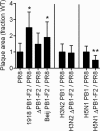
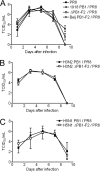
The influenza A virus PB1-F2 protein has been implicated as a virulence factor, but the mechanism by which it enhances pathogenicity is not understood. The PB1 gene segment of the H1N1 swine-origin influenza virus pandemic strain codes for a truncated PB1-F2 protein which terminates after 11 amino acids but could acquire the full-length form by mutation or reassortment. It is therefore important to understand the function and impact of this protein. We systematically assessed the effect that PB1-F2 expression has on viral polymerase activity, accumulation and localization of PB1, and replication in vitro and in mice. We used both the laboratory strain PR8 and a set of viruses engineered to study clinically relevant PB1-F2 proteins. PB1-F2 expression had modest effects on polymerase activity, PB1 accumulation, and replication that were cell type and virus strain dependent. Disruption of the PB1-F2 reading frame in a recent, seasonal H3N2 influenza virus strain did not affect these parameters, suggesting that this is not a universal function of the protein. Disruption of PB1-F2 expression in several backgrounds or expression of PB1-F2 from the 1918 pandemic strain or a 1956 H1N1 strain had no effect on viral lung loads in mice. Alternate mechanisms besides alterations to replication are likely responsible for the enhanced virulence in mammalian hosts attributed to PB1-F2 in previous studies.
Figures
References
-
- Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. - PubMed
-
- Dawood, F. S., S. Jain, L. Finelli, M. W. Shaw, S. Lindstrom, R. J. Garten, L. V. Gubareva, X. Xu, C. B. Bridges, and T. M. Uyeki. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

