The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties
- PMID: 19828672
- PMCID: PMC2785978
- DOI: 10.1104/pp.109.143388
The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties
Abstract
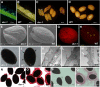
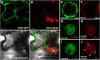
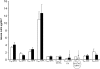
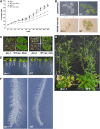
The cuticle covering every plant aerial organ is largely made of cutin that consists of fatty acids, glycerol, and aromatic monomers. Despite the huge importance of the cuticle to plant development and fitness, our knowledge regarding the assembly of the cutin polymer and its integration in the complete cuticle structure is limited. Cutin composition implies the action of acyltransferase-type enzymes that mediate polymer construction through ester bond formation. Here, we show that a member of the BAHD family of acyltransferases (DEFECTIVE IN CUTICULAR RIDGES [DCR]) is required for incorporation of the most abundant monomer into the polymeric structure of the Arabidopsis (Arabidopsis thaliana) flower cutin. DCR-deficient plants display phenotypes that are typically associated with a defective cuticle, including altered epidermal cell differentiation and postgenital organ fusion. Moreover, levels of the major cutin monomer in flowers, 9(10),16-dihydroxy-hexadecanoic acid, decreased to an almost undetectable amount in the mutants. Interestingly, dcr mutants exhibit changes in the decoration of petal conical cells and mucilage extrusion in the seed coat, both phenotypes formerly not associated with cutin polymer assembly. Excessive root branching displayed by dcr mutants and the DCR expression pattern in roots pointed to the function of DCR belowground, in shaping root architecture by influencing lateral root emergence and growth. In addition, the dcr mutants were more susceptible to salinity, osmotic, and water deprivation stress conditions. Finally, the analysis of DCR protein localization suggested that cutin polymerization, possibly the oligomerization step, is partially carried out in the cytoplasmic space. Therefore, this study extends our knowledge regarding the functionality of the cuticular layer and the formation of its major constituent the polymer cutin.
Figures





References
-
- Baum SF, Rost TL (1996) Root apical organization in Arabidopsis thaliana. 1. Root cap and protoderm. Protoplasma 192 178–188
-
- Beeckman T, De Rycke R, Viane R, Inzé D (2000) Histological study of seed coat development in Arabidopsis thaliana. J Plant Res 113 139–148
-
- Benitez JJ, Heredia-Guerrero JA, Serrano FM, Heredia A (2008) The role of hydroxyl groups in the self-assembly of long chain alkylhydroxyl carboxylic acids on mica. J Phys Chem C 112 16968–16972
-
- Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, Kunst L, Wu X, Yephremov A, Samuels L (2007) Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J 52 485–498 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

