Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium
- PMID: 19828701
- PMCID: PMC2792214
- DOI: 10.1182/blood-2009-05-220400
Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium
Abstract
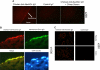
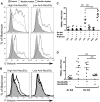
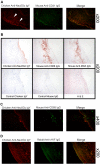
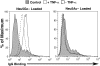
Humans are genetically unable to synthesize the common mammalian sialic acid N-glycolylneuraminic acid (Neu5Gc). However, Neu5Gc can be metabolically incorporated and covalently expressed on cultured human cell surfaces. Meanwhile, humans express varying and sometimes high titers of polyclonal anti-Neu5Gc antibodies. Here, a survey of human tissues by immunohistochemistry with both a monospecific chicken anti-Neu5Gc antibody and with affinity-purified human anti-Neu5Gc antibodies demonstrates endothelial expression of Neu5Gc, likely originating from Neu5Gc-rich foods like red meats. We hypothesized that the combination of Neu5Gc incorporation and anti-Neu5Gc antibodies can induce endothelial activation. Indeed, the incubation of high-titer human sera with Neu5Gc-fed endothelial cells led to Neu5Gc-dependent antibody binding, complement deposition, endothelial activation, selectin expression, increased cytokine secretion, and monocyte binding. The proinflammatory cytokine tumor necrosis factor-alpha also selectively enhanced human anti-Neu5Gc antibody reactivity. Anti-Neu5Gc antibodies affinity-purified from human serum also directed Neu5Gc-dependent complement deposition onto cultured endothelial cells. These data indicate a novel human-specific mechanism in which Neu5Gc-rich foods deliver immunogenic Neu5Gc to the endothelium, giving anti-Neu5Gc antibody- and complement-dependent activation, and potentially contributing to human vascular pathologies. In the case of atherosclerosis, Neu5Gc is present both in endothelium overlying plaques and in subendothelial regions, providing multiple pathways for accelerating inflammation in this disease.
Figures


References
-
- Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446(7139):1023–1029. - PubMed
-
- Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280(6):4228–4237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

