Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus
- PMID: 19828736
- PMCID: PMC2793285
- DOI: 10.1091/mbc.e09-04-0291
Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus
Abstract
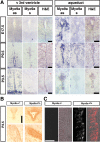
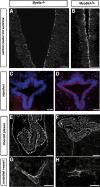
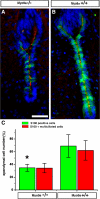
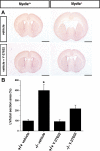
The ependymal multiciliated epithelium in the brain restricts the cerebrospinal fluid to the cerebral ventricles and regulates its flow. We report here that mice deficient for myosin IXa (Myo9a), an actin-dependent motor molecule with a Rho GTPase-activating (GAP) domain, develop severe hydrocephalus with stenosis and closure of the ventral caudal 3rd ventricle and the aqueduct. Myo9a is expressed in maturing ependymal epithelial cells, and its absence leads to impaired maturation of ependymal cells. The Myo9a deficiency further resulted in a distorted ependyma due to irregular epithelial cell morphology and altered organization of intercellular junctions. Ependymal cells occasionally delaminated, forming multilayered structures that bridged the CSF-filled ventricular space. Hydrocephalus formation could be significantly attenuated by the inhibition of the Rho-effector Rho-kinase (ROCK). Administration of ROCK-inhibitor restored maturation of ependymal cells, but not the morphological distortions of the ependyma. Similarly, down-regulation of Myo9a by siRNA in Caco-2 adenocarcinoma cells increased Rho-signaling and induced alterations in differentiation, cell morphology, junction assembly, junctional signaling, and gene expression. Our results demonstrate that Myo9a is a critical regulator of Rho-dependent and -independent signaling mechanisms that guide epithelial differentiation. Moreover, Rho-kinases may represent a new target for therapeutic intervention in some forms of hydrocephalus.
Figures



References
-
- Aijaz S., D'Atri F., Citi S., Balda M. S., Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell. 2005;8:777–786. - PubMed
-
- Assadi A. H., et al. Interaction of reelin signalling and Lis1 in brain development. Nat. Genet. 2003;3:270–276. - PubMed
-
- Bähler M. Class IX myosins. In: Coluccio L. M., editor. Myosins. Netherlands: Springer; 2008. pp. 391–401.
-
- Balda M. S., Matter K. Epithelial cell adhesion and the regulation of gene expression. Trends Cell Biol. 2003;13:310–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

