Understanding the role of DISC1 in psychiatric disease and during normal development
- PMID: 19828788
- PMCID: PMC6665304
- DOI: 10.1523/JNEUROSCI.3355-09.2009
Understanding the role of DISC1 in psychiatric disease and during normal development
Abstract
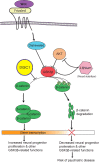
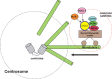
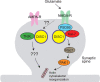
The biology of schizophrenia is complex with multiple hypotheses (dopamine, glutamate, neurodevelopmental) well supported to underlie the disease. Pathways centered on the risk factor "disrupted in schizophrenia 1" (DISC1) may be able to explain and unite these disparate hypotheses and will be the topic of this mini-symposium preview. Nearly a decade after its original identification at the center of a translocation breakpoint in a large Scottish family that was associated with major psychiatric disease, we are starting to obtain credible insights into its function and role in disease etiology. This preview will highlight a number of exciting areas of current DISC1 research that are revealing roles for DISC1 during normal brain development and also in the disease state. Together these different threads will provide a timely and exciting overview of the DISC1 field and its potential in furthering our understanding of psychiatric diseases and in developing new therapies.
Figures



References
-
- Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205. - PubMed
-
- Blaker A, DeRienzo G, Sive H. Zebrafish as a tool to study autism. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism spectrum disorders. Oxford: Oxford UP; 2009. in press.
-
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. - PubMed
-
- Brandon NJ, Handford EJ, Schurov I, Rain JC, Pelling M, Duran-Jimeniz B, Camargo LM, Oliver KR, Beher D, Shearman MS, Whiting PJ. Disrupted in Schizophrenia 1 and Nudel form a neurodevelopmentally regulated protein complex: implications for schizophrenia and other major neurological disorders. Mol Cell Neurosci. 2004;25:42–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
