The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential
- PMID: 19828790
- PMCID: PMC2879048
- DOI: 10.1523/JNEUROSCI.3657-09.2009
The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential
Abstract
The beta-amyloid (Abeta) peptide is the major constituent of amyloid plaques in Alzheimer's disease (AD) brain and is likely to play a central role in the pathogenesis of this devastating neurodegenerative disorder. The beta-secretase, beta-site amyloid precursor protein cleaving enzyme (BACE1; also called Asp2, memapsin 2), is the enzyme responsible for initiating Abeta generation. Thus, BACE is a prime drug target for the therapeutic inhibition of Abeta production in AD. Since its discovery 10 years ago, much has been learned about BACE. This review summarizes BACE properties, describes BACE translation dysregulation in AD, and discusses BACE physiological functions in sodium current, synaptic transmission, myelination, and schizophrenia. The therapeutic potential of BACE will also be considered. This is a summary of topics covered at a symposium held at the 39th annual meeting of the Society for Neuroscience and is not meant to be a comprehensive review of BACE.
Figures

 S) are also depicted connecting amino acids 216–420, 278–443, and 330–380. S-palmitoylation occurs at Cys474, Cys478, Cys482, and Cys485. Finally, a disintegrin and metalloprotease (ADAM)-like sheddase cleaves BACE1 between Ala429 and Val430 (arrow) to allow BACE1 ectodomain secretion.
S) are also depicted connecting amino acids 216–420, 278–443, and 330–380. S-palmitoylation occurs at Cys474, Cys478, Cys482, and Cys485. Finally, a disintegrin and metalloprotease (ADAM)-like sheddase cleaves BACE1 between Ala429 and Val430 (arrow) to allow BACE1 ectodomain secretion.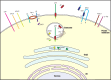
References
-
- Audenaert D, Claes L, Ceulemans B, Löfgren A, Van Broeckhoven C, De Jonghe P. A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology. 2003;61:854–856. - PubMed
-
- Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, Chrétien M, Seidah NG. Post-translational processing of β-secretase (β-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-β production. J Biol Chem. 2001;276:10879–10887. - PubMed
-
- Bennett BD, Denis P, Haniu M, Teplow DB, Kahn S, Louis JC, Citron M, Vassar R. A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer's beta-secretase. J Biol Chem. 2000;275:37712–37717. - PubMed
-
- Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, Schliebs R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer's disease beta-secretase (BACE-1) J Neural Transm. 2004;111:523–536. - PubMed
-
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1, amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
