Corticostriatal Interactions during Learning, Memory Processing, and Decision Making
- PMID: 19828796
- PMCID: PMC3849625
- DOI: 10.1523/JNEUROSCI.3177-09.2009
Corticostriatal Interactions during Learning, Memory Processing, and Decision Making
Abstract
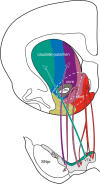
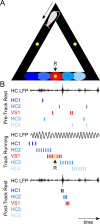
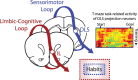
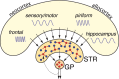
This mini-symposium aims to integrate recent insights from anatomy, behavior, and neurophysiology, highlighting the anatomical organization, behavioral significance, and information-processing mechanisms of corticostriatal interactions. In this summary of topics, which is not meant to provide a comprehensive survey, we will first review the anatomy of corticostriatal circuits, comparing different ways by which "loops" of cortical-basal ganglia circuits communicate. Next, we will address the causal importance and systems-neurophysiological mechanisms of corticostriatal interactions for memory, emphasizing the communication between hippocampus and ventral striatum during contextual conditioning. Furthermore, ensemble recording techniques have been applied to compare information processing in the dorsal and ventral striatum to predictions from reinforcement learning theory. We will next discuss how neural activity develops in corticostriatal areas when habits are learned. Finally, we will evaluate the role of GABAergic interneurons in dynamically transforming cortical inputs into striatal output during learning and decision making.
Figures

References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. - PubMed
-
- Aldridge JW, Berridge KC, Rosen AR. Basal ganglia neural mechanisms of natural movement sequences. Can J Physiol Pharmacol. 2004;82:732–739. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. - PubMed
-
- Atallah HE, Lopez-Paniagua D, Rudy JW, O'Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci. 2007;10:126–131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
