Modulation of perineuronal nets and parvalbumin with developmental song learning
- PMID: 19828802
- PMCID: PMC2769505
- DOI: 10.1523/JNEUROSCI.2974-09.2009
Modulation of perineuronal nets and parvalbumin with developmental song learning
Abstract
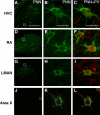
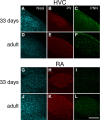
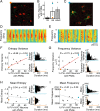
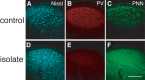
Neural circuits and behavior are shaped during developmental phases of maximal plasticity known as sensitive or critical periods. Neural correlates of sensory critical periods have been identified, but their roles remain unclear. Factors that define critical periods in sensorimotor circuits and behavior are not known. Birdsong learning in the zebra finch occurs during a sensitive period similar to that for human speech. We now show that perineuronal nets, which correlate with sensory critical periods, surround parvalbumin-positive neurons in brain areas that are dedicated to singing. The percentage of both total and parvalbumin-positive neurons with perineuronal nets increased with development. In HVC (this acronym is the proper name), a song area important for sensorimotor integration, the percentage of parvalbumin neurons with perineuronal nets correlated with song maturity. Shifting the vocal critical period with tutor song deprivation decreased the percentage of neurons that were parvalbumin positive and the relative staining intensity of both parvalbumin and a component of perineuronal nets. Developmental song learning shares key characteristics with sensory critical periods, suggesting shared underlying mechanisms.
Figures

References
-
- Belmonte MK, Cook EH, Jr, Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powell SB, Levitt PR, Perry EK, Jiang YH, DeLorey TM, Tierney E. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. - PubMed
-
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. - PubMed
-
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) J Neurobiol. 2006;66:1044–1060. - PubMed
-
- Braun K, Scheich H, Zuschratter W, Heizmann CW, Matute C, Streit P. Postnatal development of parvalbumin-, calbindin- and adult GABA-immunoreactivity in two visual nuclei of zebra finches. Brain Res. 1988;475:205–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
