Cerebellar contributions to adaptive control of saccades in humans
- PMID: 19828807
- PMCID: PMC2994243
- DOI: 10.1523/JNEUROSCI.3115-09.2009
Cerebellar contributions to adaptive control of saccades in humans
Abstract
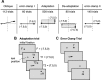
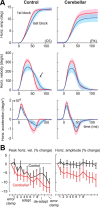
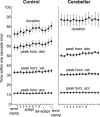
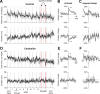
The cerebellum may monitor motor commands and through internal feedback correct for anticipated errors. Saccades provide a test of this idea because these movements are completed too quickly for sensory feedback to be useful. Earlier, we reported that motor commands that accelerate the eyes toward a constant amplitude target showed variability. Here, we demonstrate that this variability is not random noise, but is due to the cognitive state of the subject. Healthy people showed within-saccade compensation for this variability with commands that arrived later in the same saccade. However, in people with cerebellar damage, the same variability resulted in dysmetria. This ability to correct for variability in the motor commands that initiated a saccade was a predictor of each subject's ability to learn from endpoint errors. In a paradigm in which a target on the horizontal meridian jumped vertically during the saccade (resulting in an endpoint error), the adaptive response exhibited two timescales: a fast timescale that learned quickly from endpoint error but had poor retention, and a slow timescale that learned slowly but had strong retention. With cortical cerebellar damage, the fast timescale of adaptation was effectively absent, but the slow timescale was less impaired. Therefore, the cerebellum corrects for variability in the motor commands that initiate saccades within the same movement via an adaptive response that not only exhibits strong sensitivity to previous endpoint errors, but also rapid forgetting.
Figures


References
-
- Boehnke SE, Berg DJ, Marino RA, Itti L, Munoz DP. Adaptation, habituation and dishabituation of visual responses in the superior colliculus. Soc Neurosci Abs. 2007 617.12/PP14.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
