gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment
- PMID: 19828817
- PMCID: PMC6665297
- DOI: 10.1523/JNEUROSCI.2362-09.2009
gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment
Abstract
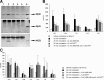
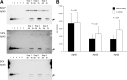
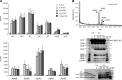
Amyloid beta protein (Abeta), a pathogenic molecule associated with Alzheimer's disease, is produced by gamma-secretase, which cleaves the beta-carboxyl terminal fragment (betaCTF) of beta-amyloid precursor protein in the middle of its transmembrane domain. How the cleavage proceeds within the membrane has long been enigmatic. We hypothesized previously that betaCTF is cleaved first at the membrane-cytoplasm boundary, producing two long Abetas, Abeta(48) and Abeta(49), which are processed further by releasing three residues at each step to produce Abeta(42) and Abeta(40), respectively. To test this hypothesis, we used liquid chromatography tandem mass spectrometry (LC-MS/MS) to quantify the specific tripeptides that are postulated to be released. Using CHAPSO (3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxyl-1-propanesulfonate)-reconstituted gamma-secretase system, we confirmed that Abeta(49) is converted to Abeta(43/40) by successively releasing two or three tripeptides and that Abeta(48) is converted to Abeta(42/38) by successively releasing two tripeptides or these plus an additional tetrapeptide. Most unexpectedly, LC-MS/MS quantification revealed an induction period, 3-4 min, in the generation of peptides. When extrapolated, each time line for each tripeptide appears to intercept the same point on the x-axis. According to numerical simulation based on the successive reaction kinetics, the induction period exists. These results strongly suggest that Abeta is generated through the stepwise processing of betaCTF by gamma-secretase.
Figures





References
-
- Beher D, Wrigley JD, Owens AP, Shearman MS. Generation of C-terminally truncated amyloid-β peptides is dependent on γ-secretase activity. J Neurochem. 2002;82:563–575. - PubMed
-
- Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y. Truncated carboxyl-terminal fragments of β-amyloid precursor protein are processed to amyloid β-proteins 40 and 42. Biochemistry. 2004;43:13532–13540. - PubMed
-
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ 42(43) and Aβ 40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ 42(43) Neuron. 1994;13:45–53. - PubMed
-
- Kakuda N, Funamoto S, Yagishita S, Takami M, Osawa S, Dohmae N, Ihara Y. Equimolar production of amyloid β-protein and amyloid precursor protein intracellular domain from β-carboxyl-terminal fragment by γ-secretase. J Biol Chem. 2006;281:14776–14786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
