Safety and tolerability of putaminal AADC gene therapy for Parkinson disease
- PMID: 19828868
- PMCID: PMC2839805
- DOI: 10.1212/WNL.0b013e3181c29356
Safety and tolerability of putaminal AADC gene therapy for Parkinson disease
Abstract
Background: In Parkinson disease (PD), the benefit of levodopa therapy becomes less marked over time, perhaps because degeneration of nigrostrial neurons causes progressive loss of aromatic l-amino acid decarboxylase (AADC), the enzyme that converts levodopa into dopamine. In a primate model of PD, intrastriatal infusion of an adeno-associated viral type 2 vector containing the human AADC gene (AAV-hAADC) results in robust response to low-dose levodopa without the side effects associated with higher doses. These data prompted a clinical trial.
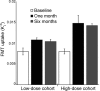
Methods: Patients with moderately advanced PD received bilateral intraputaminal infusion of AAV-hAADC vector. Low-dose and high-dose cohorts (5 patients in each) were studied using standardized clinical rating scales at baseline and 6 months. PET scans using the AADC tracer [(18)F]fluoro-L-m-tyrosine (FMT) were performed as a measure of gene expression.
Results: The gene therapy was well tolerated, but 1 symptomatic and 2 asymptomatic intracranial hemorrhages followed the operative procedure. Total and motor rating scales improved in both cohorts. Motor diaries also showed increased on-time and reduced off-time without increased "on" time dyskinesia. At 6 months, FMT PET showed a 30% increase of putaminal uptake in the low-dose cohort and a 75% increase in the high-dose cohort.
Conclusion: This study provides class IV evidence that bilateral intrastriatal infusion of adeno-associated viral type 2 vector containing the human AADC gene improves mean scores on the Unified Parkinson's Disease Rating Scale by approximately 30% in the on and off states, but the surgical procedure may be associated with an increased risk of intracranial hemorrhage and self-limited headache.
Figures


References
-
- Nagatsu T, Sawada M. Biochemistry of postmortem brains in Parkinson’s disease: historical overview and future prospects. J Neural Transm Suppl 2007;113–120. - PubMed
-
- Bankiewicz KS, Forsayeth J, Eberling JL, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther 2006;14:564–570. - PubMed
-
- Bankiewicz KS, Eberling JL, Kohutnicka M, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys: in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol 2000;164:2–14. - PubMed
-
- Eberling JL, Jagust WJ, Christine CW, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 2008;70:1980–1983. - PubMed
-
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician J Psychiatr Res 1975;12:189–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA135358/CA/NCI NIH HHS/United States
- R01 CA94253-01/CA/NCI NIH HHS/United States
- R01 NS37167/NS/NINDS NIH HHS/United States
- R41 AG030241/AG/NIA NIH HHS/United States
- AG024904/AG/NIA NIH HHS/United States
- 5 U10 NS044460/NS/NINDS NIH HHS/United States
- R01 CA119414/CA/NCI NIH HHS/United States
- 5U10NS044460/NS/NINDS NIH HHS/United States
- R01 CA135626/CA/NCI NIH HHS/United States
- AG027859/AG/NIA NIH HHS/United States
- U54 CA90788/CA/NCI NIH HHS/United States
- R01 EB000482-01/EB/NIBIB NIH HHS/United States
- AG027984/AG/NIA NIH HHS/United States
- R01NS046487/NS/NINDS NIH HHS/United States
- S10 RR023051/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
