Intracellular dynamics of hippocampal place cells during virtual navigation
- PMID: 19829374
- PMCID: PMC2771429
- DOI: 10.1038/nature08499
Intracellular dynamics of hippocampal place cells during virtual navigation
Abstract
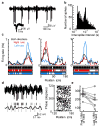
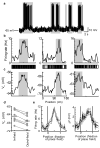
Hippocampal place cells encode spatial information in rate and temporal codes. To examine the mechanisms underlying hippocampal coding, here we measured the intracellular dynamics of place cells by combining in vivo whole-cell recordings with a virtual-reality system. Head-restrained mice, running on a spherical treadmill, interacted with a computer-generated visual environment to perform spatial behaviours. Robust place-cell activity was present during movement along a virtual linear track. From whole-cell recordings, we identified three subthreshold signatures of place fields: an asymmetric ramp-like depolarization of the baseline membrane potential, an increase in the amplitude of intracellular theta oscillations, and a phase precession of the intracellular theta oscillation relative to the extracellularly recorded theta rhythm. These intracellular dynamics underlie the primary features of place-cell rate and temporal codes. The virtual-reality system developed here will enable new experimental approaches to study the neural circuits underlying navigation.
Figures


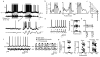
Comment in
-
Neuroscience: The inside story on place cells.Nature. 2009 Oct 15;461(7266):889-90. doi: 10.1038/461889a. Nature. 2009. PMID: 19829361 No abstract available.
-
Neuroscience in a virtual world.Nat Methods. 2010 Dec;7(12):948-9. doi: 10.1038/nmeth1210-948a. Nat Methods. 2010. PMID: 21158013 No abstract available.
References
-
- Moser EI, Kropff E, Moser MB. Place Cells, Grid Cells, and the Brain’s Spatial Representation System. Annu Rev Neurosci. 2008;31:69–89. - PubMed
-
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. - PubMed
-
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. - PubMed
-
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

