Scaleable catalytic asymmetric Strecker syntheses of unnatural alpha-amino acids
- PMID: 19829379
- PMCID: PMC2778849
- DOI: 10.1038/nature08484
Scaleable catalytic asymmetric Strecker syntheses of unnatural alpha-amino acids
Abstract
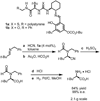
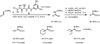
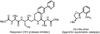
Alpha-amino acids are the building blocks of proteins and are widely used as components of medicinally active molecules and chiral catalysts. Efficient chemo-enzymatic methods for the synthesis of enantioenriched alpha-amino acids have been developed, but it is still a challenge to obtain non-natural amino acids. Alkene hydrogenation is broadly useful for the enantioselective catalytic synthesis of many classes of amino acids, but it is not possible to obtain alpha-amino acids bearing aryl or quaternary alkyl alpha-substituents using this method. The Strecker synthesis-the reaction of an imine or imine equivalent with hydrogen cyanide, followed by nitrile hydrolysis-is an especially versatile chemical method for the synthesis of racemic alpha-amino acids. Asymmetric Strecker syntheses using stoichiometric amounts of a chiral reagent have been applied successfully on gram-to-kilogram scales, yielding enantiomerically enriched alpha-amino acids. In principle, Strecker syntheses employing sub-stoichiometric quantities of a chiral reagent could provide a practical alternative to these approaches, but the reported catalytic asymmetric methods have seen limited use on preparative scales (more than a gram). The limited utility of existing catalytic methods may be due to several important factors, including the relatively complex and precious nature of the catalysts and the requisite use of hazardous cyanide sources. Here we report a new catalytic asymmetric method for the syntheses of highly enantiomerically enriched non-natural amino acids using a simple chiral amido-thiourea catalyst to control the key hydrocyanation step. This catalyst is robust, without sensitive functional groups, so it is compatible with aqueous cyanide salts, which are safer and easier to handle than other cyanide sources; this makes the method adaptable to large-scale synthesis. We have used this new method to obtain enantiopure amino acids that are not readily prepared by enzymatic methods or by chemical hydrogenation.
Figures
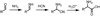
References
-
- Bhat SV, Nagasampagi BA, Sivakumar M, editors. Chemistry of Natural Products. Narosa: Springer; 2005. pp. 317–393.
-
- Wang L, Shultz PG. Expanding the genetic code. Angew. Chem., Int. Ed. 2005;44:34–66. - PubMed
-
- Tsantrizos YS. Peptidomimetic therapeutic agents targeting the protease enzyme of the human immunodeficiency virus and hepatitis C virus. Acc. Chem. Res. 2008;41:1252–1263. - PubMed
-
- Davie EAC, Mennen SM, Xu YJ, Miller SJ. Asymmetric catalysis mediated by synthetic peptides. Chem. Rev. 2007;107:5759–5812. - PubMed
-
- Helmchen G, Pfaltz A. Phosphinooxazolines—a new class of versatile, modular P,N-ligands for asymmetric catalysis. Acc. Chem. Res. 2000;33:336–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

