Galectin-3 is associated with prostasomes in human semen
- PMID: 19830550
- PMCID: PMC3261635
- DOI: 10.1007/s10719-009-9262-9
Galectin-3 is associated with prostasomes in human semen
Abstract
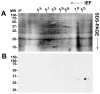
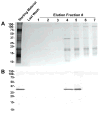
Galectin-3 is a beta-galactoside-binding protein involved in immunomodulation, cell interactions, cancer progression, and pathogenesis of infectious organisms. We report the identification and characterization of galectin-3 in human semen. In the male reproductive tract, the approximately 30 kDa galectin-3 protein was identified in testis, epididymis, vas deferens, prostate, seminal vesicle, and sperm protein extracts. In seminal plasma, galectin-3 was identified in the soluble fraction and in prostasomes, cholesterol-rich, membranous vesicles that are secreted by the prostate and incorporated into seminal plasma during ejaculation. Two-dimensional immunoblot analysis of purified prostasomes identified five galectin-3 isoelectric variants with a pI range of 7.0 to 9.2. Affinity purification and tandem mass spectrometry of beta-galactoside-binding proteins from prostasomes confirmed the presence of galectin-3 in prostasomes and identified a truncated galectin-3 variant. The intact galectin-3 molecule contains a carbohydrate recognition domain and a non-lectin domain that interacts with protein and lipid moieties. The identification of a monovalent galectin-3 fragment with conserved carbohydrate-binding activity indicates the functional relevance of this truncation and suggests a regulatory mechanism for galectin-3 in prostasomes. Surface biotinylation studies suggested that galectin-3 and the truncated galectin-3 variant are localized to the prostasome surface. Prostasomes are proposed to function in immunosuppression and regulation of sperm function in the female reproductive tract, are implicated in facilitating sexually-transmitted infections, and are indicated in prostate cancer progression. Given the overlap in functional significance, the identification of galectin-3 in prostasomes lays the groundwork for future studies of galectin-3 and prostasomes in reproduction, disease transmission, and cancer progression.
Figures







References
-
- Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–35. - PubMed
-
- Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–52. - PubMed
-
- Bachhawat-Sikder K, Thomas CJ, Surolia A. Thermodynamic analysis of the binding of galactose and poly-N-acetyllactosamine derivatives to human galectin-3. FEBS Lett. 2001;500:75–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

