The interaction of Thrombospondins with extracellular matrix proteins
- PMID: 19830595
- PMCID: PMC2778591
- DOI: 10.1007/s12079-009-0074-2
The interaction of Thrombospondins with extracellular matrix proteins
Abstract
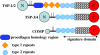
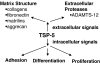
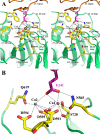
The thrombospondins (TSPs) are a family of five matricellular proteins that appear to function as adapter molecules to guide extracellular matrix synthesis and tissue remodeling in a variety of normal and disease settings. Various TSPs have been shown to bind to fibronectin, laminin, matrilins, collagens and other extracellular matrix (ECM) proteins. The importance of TSP-1 in this context is underscored by the fact that it is rapidly deposited at the sites of tissue damage by platelets. An association of TSPs with collagens has been known for over 25 years. The observation that the disruption of the TSP-2 gene in mice leads to collagen fibril abnormalities provided important in vivo evidence that these interactions are physiologically important. Recent biochemical studies have shown that TSP-5 promotes collagen fibril assembly and structural studies suggest that TSPs may interact with collagens through a highly conserved potential metal ion dependent adhesion site (MIDAS). These interactions are critical for normal tissue homeostasis, tumor progression and the etiology of skeletal dysplasias.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

