Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets
- PMID: 19830596
- PMCID: PMC2866992
- DOI: 10.1007/s12192-009-0148-3
Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets
Abstract
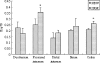
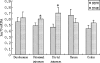
The objective of this study is to investigate the expression and distribution of heat shock protein 70 (Hsp70) in the intestine of intrauterine growth retardation (IUGR) piglets. Samples from the duodenum, prejejunum, distal jejunum, ileum, and colon of IUGR and normal-body-weight (NBW) piglets were collected at birth. The results indicated that the body and intestine weight of IUGR piglets were significantly lower than NBW piglets. The villus height and villus/crypt ratio in jejunum and ileum of IUGR piglets were significantly reduced compared to NBW piglets. These results indicated that IUGR causes abnormal gastrointestinal morphologies and gastrointestinal dysfunction. The mRNA of hsp70 was increased in prejejunum (P < 0.05), distal jejunum (P < 0.05), and colon in IUGR piglets. However, the hsp70 mRNA in ileum of piglets with IUGR was decreased. Similar to hsp70 mRNA, the protein levels of Hsp70 in prejejunum (P < 0.05), distal jejunum, and colon (P < 0.05) in IUGR piglets were higher than those in NBW piglets. These results indicated that the expression of Hsp70 in the intestinal piglets was upregulated by IUGR, and different intestinal sites had different responses to stress. Meanwhile, the localization of Hsp70 in the epithelial cells of the whole villi and intestinal gland rather than in the lamina propria and myenteron suggested that Hsp70 has a cytoprotective role in epithelial cell function and structure.
Figures


References
-
- Ahmad RH, Craig CC, Arlan R. Expression of heat shock genes in hepatocytes is affected by age and food restriction in rats. J Nutri. 1995;125:410–418. - PubMed
-
- Bao ED, Sultan KR, Nowak B, Hartung J. Localization of heat shock proteins and histopathological changes in the kidneys of transported pigs. Livest Sci. 2008;118(3):231–237. doi: 10.1016/j.livsci.2008.01.028. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

