HIV gag protein is efficiently cross-presented when targeted with an antibody towards the DEC-205 receptor in Flt3 ligand-mobilized murine DC
- PMID: 19830741
- PMCID: PMC2933266
- DOI: 10.1002/eji.200939748
HIV gag protein is efficiently cross-presented when targeted with an antibody towards the DEC-205 receptor in Flt3 ligand-mobilized murine DC
Abstract
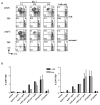
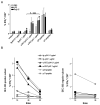
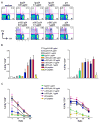
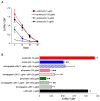
DC present exogenous proteins to MHC class I-restricted CD8+ T cells. This function does not require endogenous antigen synthesis within DC, providing the potential to elicit CD8+ T-cell responses to immune complexes, inactivated microbes, dying cells, and proteins such as OVA. In mice, the CD8+ or DEC-205+ DC are specialized for cross-presentation, and this subset can be increased 10-fold in numbers following Fms-like tyrosine kinase 3 ligand (Flt3L) treatment in vivo. Therefore, we studied cross-presentation by abundant Flt3L DC using HIV gag protein. When enriched by positive selection with anti-CD11c beads, cells from Flt3L mice are not only more abundant but are also more highly enriched in CD11chigh DC, particularly the DEC-205+ subset. DC cross-present HIV gag to primed CD8+ T cells, but when the antigen is delivered within an antibody to DEC-205 receptor, cross-presentation becomes 100-fold more efficient than non-targeted antigen. This finding requires gag to be engineered into anti-DEC antibody, not just mixed with antibody. Flt3L DC are a valuable tool to study cross-presentation, since their use overcomes the obstacle posed by the low number of cross-presenting DC in the steady state. These findings support future experiments to use Flt3L to enhance presentation of DC-targeted vaccines.
Conflict of interest statement
Figures
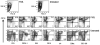
References
-
- Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. - PubMed
-
- Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

