Fear learning and extinction are linked to neuronal plasticity through Rin1 signaling
- PMID: 19830836
- PMCID: PMC2864017
- DOI: 10.1002/jnr.22252
Fear learning and extinction are linked to neuronal plasticity through Rin1 signaling
Abstract
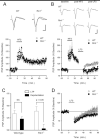
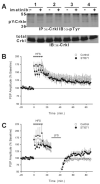
The amygdala is known to have a crucial role in both the acquisition and extinction of conditioned fear, but the physiological changes and biochemical mechanisms underlying these forms of learning are only partly understood. The Ras effector Rin1 activates Abl tyrosine kinases and Rab5 GTPases and is highly expressed in mature neurons of the telencephalon including the amygdala, where it inhibits the acquisition of fear memories (Rin1(-/-) mice show enhanced learning of conditioned fear). Here we report that Rin1(-/-) mice exhibit profound deficits in both latent inhibition and fear extinction, suggesting a critical role for Rin1 in gating the acquisition and persistence of cue-dependent fear conditioning. Surprisingly, we also find that depotentiation, a proposed cellular mechanism of extinction, is enhanced at lateral-basolateral (LA-BLA) amygdaloid synapses in Rin1(-/-) mice. Inhibition of a single Rin1 downstream effector pathway, the Abl tyrosine kinases, led to reduced amygdaloid depotentiation, arguing that proper coordination of Abl and Rab5 pathways is critical for Rin1-mediated effects on plasticity. While demonstrating a correlation between amygdala plasticity and fear learning, our findings argue against models proposing a direct causative relationship between amygdala depotentiation and fear extinction. Taken together, the behavior and physiology of Rin1(-/-) mice provide new insights into the regulation of memory acquisition and maintenance. In addition, Rin1(-/-) mice should prove useful as a model for pathologies marked by enhanced fear acquisition and retention, such as posttraumatic stress disorder.
Figures




References
-
- Barbieri MA, Kong C, Chen PI, Horazdovsky BF, Stahl PD. The SRC homology 2 domain of Rin1 mediates its binding to the epidermal growth factor receptor and regulates receptor endocytosis. J Biol Chem. 2003;278:32027–32036. - PubMed
-
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. - PubMed
-
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

