The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat
- PMID: 19831719
- PMCID: PMC2864458
- DOI: 10.1089/neu.2008.0782
The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat
Abstract
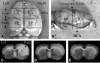
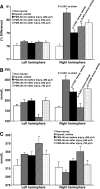
The protein kinase C activator phorbol 12-myristate 13-acetate (PMA) is known to interact with aquaporin 4 (AQP 4), a water-selective transporting protein that is abundant in astrocytes, and has experimentally been found to decrease osmotically-induced cell swelling. The purpose of this study was to examine whether PMA reduces brain edema following focal ischemia induced by middle cerebral artery (MCA) occlusion by modulation of AQP4 expression. Male Sprague-Dawley rats were randomly assigned to either sham surgery (n = 6), or a continuous intravenous infusion of vehicle (1% dimethylsulfoxide), followed by MCA occlusion (n = 18), and administration of PMA at 50 microg/kg (n = 6) or at 200 microg/kg (n = 6) starting 60 min before or 30 min (200 microg/kg; n = 6) or 60 min (200 microg/kg; n = 6) after MCA occlusion. Cerebral blood flow was monitored with laser Doppler over the MCA territory, and confirmed a 70% reduction during occlusion. After a 2-h period of ischemia and 2 h of reperfusion, the animals were sacrificed for assessment of brain water content and sodium and potassium concentration. AQP4 expression was assessed by immunoblotting and quantified by densitometry (n = 24). Statistical analysis was performed by ANOVA followed by Tukey's post-hoc test. PMA treatment at 200 microg/kg significantly reduced brain water concentration in the infarcted area when started 60 min before or 30 min after occlusion (p < 0.001 and p = 0.022, respectively), and prevented the subsequent sodium shift (p < 0.05). PMA normalized the AQP4 upregulation in ischemia (p = 0.021). A downregulation of AQP4 in the ischemic area paralleling the reduction in brain edema formation following PMA treatment suggests that the effect was mediated by AQP4 modulation.
Figures



Similar articles
-
Modulation of AQP4 expression by the protein kinase C activator, phorbol myristate acetate, decreases ischemia-induced brain edema.Acta Neurochir Suppl. 2006;96:393-7. doi: 10.1007/3-211-30714-1_81. Acta Neurochir Suppl. 2006. PMID: 16671492
-
The modulation of aquaporin-4 by using PKC-activator (phorbol myristate acetate) and V1a receptor antagonist (SR49059) following middle cerebral artery occlusion/reperfusion in the rat.Acta Neurochir Suppl. 2008;102:431-6. doi: 10.1007/978-3-211-85578-2_84. Acta Neurochir Suppl. 2008. PMID: 19388361
-
Propofol inhibits aquaporin 4 expression through a protein kinase C-dependent pathway in an astrocyte model of cerebral ischemia/reoxygenation.Anesth Analg. 2009 Nov;109(5):1493-9. doi: 10.1213/ANE.0b013e3181b893f3. Anesth Analg. 2009. PMID: 19843787
-
Remote ischemic post-conditioning improves neurological function by AQP4 down-regulation in astrocytes.Behav Brain Res. 2015 Aug 1;289:1-8. doi: 10.1016/j.bbr.2015.04.024. Epub 2015 Apr 21. Behav Brain Res. 2015. PMID: 25907740 Review.
-
Role of aquaporin-4 in cerebral edema and stroke.Handb Exp Pharmacol. 2009;(190):159-70. doi: 10.1007/978-3-540-79885-9_7. Handb Exp Pharmacol. 2009. PMID: 19096776 Free PMC article. Review.
Cited by
-
Progress in AQP Research and New Developments in Therapeutic Approaches to Ischemic and Hemorrhagic Stroke.Int J Mol Sci. 2016 Jul 18;17(7):1146. doi: 10.3390/ijms17071146. Int J Mol Sci. 2016. PMID: 27438832 Free PMC article. Review.
-
The Laminin-Induced Phosphorylation of PKCδ Regulates AQP4 Distribution and Water Permeability in Rat Astrocytes.Cell Mol Neurobiol. 2021 Nov;41(8):1743-1757. doi: 10.1007/s10571-020-00944-w. Epub 2020 Aug 26. Cell Mol Neurobiol. 2021. PMID: 32851539 Free PMC article.
-
Role of Matrix Metalloproteinases in the Pathogenesis of Traumatic Brain Injury.Mol Neurobiol. 2016 Nov;53(9):6106-6123. doi: 10.1007/s12035-015-9520-8. Epub 2015 Nov 5. Mol Neurobiol. 2016. PMID: 26541883 Free PMC article. Review.
-
Propofol Protects the Blood-Brain Barrier After Traumatic Brain Injury by Stabilizing the Extracellular Matrix via Prrx1: From Neuroglioma to Neurotrauma.Neurochem Res. 2024 Oct;49(10):2743-2762. doi: 10.1007/s11064-024-04202-z. Epub 2024 Jul 1. Neurochem Res. 2024. PMID: 38951281
-
DOR activation inhibits anoxic/ischemic Na+ influx through Na+ channels via PKC mechanisms in the cortex.Exp Neurol. 2012 Aug;236(2):228-39. doi: 10.1016/j.expneurol.2012.05.006. Epub 2012 May 15. Exp Neurol. 2012. PMID: 22609332 Free PMC article.
References
-
- Belayev L. Alonso O.F. Busto R. Zhao W. Ginsberg M.D. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. ; discussion 1623. - PubMed
-
- Chen Y. Swanson R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003;23:137–149. - PubMed
-
- Chesnut R.M. Marshall L.F. Klauber M.R. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. - PubMed
-
- Dijkhuizen R.M. de Graaf R.A. Tulleken K.A. Nicolay K. Changes in the diffusion of water and intracellular metabolites after excitotoxic injury and global ischemia in neonatal rat brain. J. Cereb Blood Flow Metab. 1999;19:341–349. - PubMed
-
- Fu X. Li Q. Feng Z. Mu D. The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia. 2007;55:935–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

