Population diversity and antibody selective pressure to Plasmodium falciparum MSP1 block2 locus in an African malaria-endemic setting
- PMID: 19832989
- PMCID: PMC2770483
- DOI: 10.1186/1471-2180-9-219
Population diversity and antibody selective pressure to Plasmodium falciparum MSP1 block2 locus in an African malaria-endemic setting
Abstract
Background: Genetic evidence for diversifying selection identified the Merozoite Surface Protein1 block2 (PfMSP1 block2) as a putative target of protective immunity against Plasmodium falciparum. The locus displays three family types and one recombinant type, each with multiple allelic forms differing by single nucleotide polymorphism as well as sequence, copy number and arrangement variation of three amino acid repeats. The family-specific antibody responses observed in endemic settings support immune selection operating at the family level. However, the factors contributing to the large intra-family allelic diversity remain unclear. To address this question, population allelic polymorphism and sequence variant-specific antibody responses were studied in a single Senegalese rural community where malaria transmission is intense and perennial.
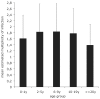
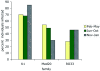
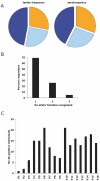
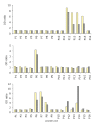
Results: Family distribution showed no significant temporal fluctuation over the 10 y period surveyed. Sequencing of 358 PCR fragments identified 126 distinct alleles, including numerous novel alleles in each family and multiple novel alleles of recombinant types. The parasite population consisted in a large number of low frequency alleles, alongside one high-frequency and three intermediate frequency alleles. Population diversity tests supported positive selection at the family level, but showed no significant departure from neutrality when considering intra-family allelic sequence diversity and all families combined. Seroprevalence, analysed using biotinylated peptides displaying numerous sequence variants, was moderate and increased with age. Reactivity profiles were individual-specific, mapped to the family-specific flanking regions and to repeat sequences shared by numerous allelic forms within a family type. Seroreactivity to K1-, Mad20- and R033 families correlated with the relative family genotype distribution within the village. Antibody specificity remained unchanged with cumulated exposure to an increasingly large number of alleles.
Conclusion: The Pfmsp1 block2 locus presents a very large population sequence diversity. The lack of stable acquisition of novel antibody specificities despite exposure to novel allelic forms is reminiscent of clonal imprinting. The locus appears under antibody-mediated diversifying selection in a variable environment that maintains a balance between the various family types without selecting for sequence variant allelic forms. There is no evidence of positive selection for intra-family sequence diversity, consistent with the observed characteristics of the antibody response.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

