The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse
- PMID: 19833087
- PMCID: PMC2791175
- DOI: 10.1016/j.immuni.2009.08.024
The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse
Abstract
Killing by cytotoxic T lymphocytes (CTLs) is mediated by the secretion of lytic granules. The centrosome plays a key role in granule delivery, polarizing to the central supramolecular activation complex (cSMAC) within the immunological synapse upon T cell receptor (TCR) activation. Although stronger TCR signals lead to increased target cell death than do weaker signals, it is not known how the strength of TCR signal controls polarization of the centrosome and lytic granules. By using TCR transgenic OT-I CTLs, we showed that both high- and low-avidity interactions led to centrosome polarization to the cSMAC. However, only high-avidity interactions, which induced a higher threshold of intracellular signaling, gave rise to granule recruitment to the polarized centrosome at the synapse. By controlling centrosome and granule polarization independently, the centrosome is able to respond rapidly to weak signals so that CTLs are poised and ready for the trigger for granule delivery.
Figures

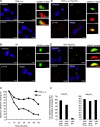
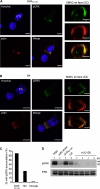

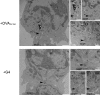
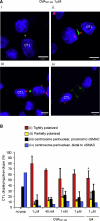

Comment in
-
A view to a kill: how ligand quality controls lethal hits.Immunity. 2009 Oct 16;31(4):531-3. doi: 10.1016/j.immuni.2009.09.011. Immunity. 2009. PMID: 19833082
References
-
- al-Ramadi B.K., Jelonek M.T., Boyd L.F., Margulies D.H., Bothwell A.L. Lack of strict correlation of functional sensitization with the apparent affinity of MHC/peptide complexes for the TCR. J. Immunol. 1995;155:662–673. - PubMed
-
- Alam S.M., Travers P.J., Wung J.L., Nasholds W., Redpath S., Jameson S.C., Gascoigne N.R. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. - PubMed
-
- Alam S.M., Davies G.M., Lin C.M., Zal T., Nasholds W., Jameson S.C., Hogquist K.A., Gascoigne N.R., Travers P.J. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. - PubMed
-
- Alcover A., Alarcon B. Internalization and intracellular fate of TCR-CD3 complexes. Crit. Rev. Immunol. 2000;20:325–346. - PubMed
-
- Boerth N.J., Judd B.A., Koretzky G.A. Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J. Biol. Chem. 2000;275:5143–5152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

