Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain
- PMID: 19833088
- PMCID: PMC2778196
- DOI: 10.1016/j.immuni.2009.09.004
Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain
Abstract
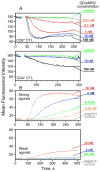
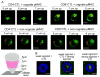
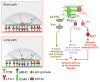
Cytolytic granules mediate killing of virus-infected cells by cytotoxic T lymphocytes. We show here that the granules can take long or short paths to the secretory domain. Both paths utilized the same intracellular molecular events, which have different spatial and temporal arrangements and are regulated by the kinetics of Ca(2+)-mediated signaling. Rapid signaling caused swift granule concentration near the microtubule-organizing center (MTOC) and subsequent delivery by the polarized MTOC directly to the secretory domain-the shortest path. Indolent signaling led to late recruitment of granules that moved along microtubules to the periphery of the synapse and then moved tangentially to fuse at the outer edge of the secretory domain-a longer path. The short pathway is associated with faster granule release and more efficient killing than the long pathway. Thus, the kinetics of early signaling regulates the quality of the T cell cytolytic response.
Figures




Comment in
-
A view to a kill: how ligand quality controls lethal hits.Immunity. 2009 Oct 16;31(4):531-3. doi: 10.1016/j.immuni.2009.09.011. Immunity. 2009. PMID: 19833082
Similar articles
-
A view to a kill: how ligand quality controls lethal hits.Immunity. 2009 Oct 16;31(4):531-3. doi: 10.1016/j.immuni.2009.09.011. Immunity. 2009. PMID: 19833082
-
The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse.Immunity. 2009 Oct 16;31(4):621-31. doi: 10.1016/j.immuni.2009.08.024. Immunity. 2009. PMID: 19833087 Free PMC article.
-
An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse.Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):6073-8. doi: 10.1073/pnas.1218640110. Epub 2013 Mar 27. Proc Natl Acad Sci U S A. 2013. PMID: 23536289 Free PMC article.
-
Mechanisms controlling granule-mediated cytolytic activity of cytotoxic T lymphocytes.Immunol Res. 2011 Dec;51(2-3):183-94. doi: 10.1007/s12026-011-8252-8. Immunol Res. 2011. PMID: 22058021 Free PMC article. Review.
-
Molecular regulation of the plasma membrane-proximal cellular steps involved in NK cell cytolytic function.J Cell Sci. 2020 Feb 21;133(5):jcs240424. doi: 10.1242/jcs.240424. J Cell Sci. 2020. PMID: 32086255 Free PMC article. Review.
Cited by
-
In Vivo Motility Patterns Displayed by Immune Cells Under Inflammatory Conditions.Front Immunol. 2022 Jan 3;12:804159. doi: 10.3389/fimmu.2021.804159. eCollection 2021. Front Immunol. 2022. PMID: 35046959 Free PMC article. Review.
-
Desensitized chimeric antigen receptor T cells selectively recognize target cells with enhanced antigen expression.Nat Commun. 2018 Feb 1;9(1):468. doi: 10.1038/s41467-018-02912-x. Nat Commun. 2018. PMID: 29391449 Free PMC article.
-
Molecular mechanisms and functional implications of polarized actin remodeling at the T cell immunological synapse.Cell Mol Life Sci. 2015 Feb;72(3):537-556. doi: 10.1007/s00018-014-1760-7. Epub 2014 Oct 30. Cell Mol Life Sci. 2015. PMID: 25355055 Free PMC article. Review.
-
HIV-1 Virological Synapse is not Simply a Copycat of the Immunological Synapse.Viruses. 2010 May 1;2(5):1239-60. doi: 10.3390/v2051239. Viruses. 2010. PMID: 20890395 Free PMC article.
-
Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment.Mol Biol Cell. 2010 Jul 1;21(13):2241-56. doi: 10.1091/mbc.e09-11-0930. Epub 2010 May 5. Mol Biol Cell. 2010. PMID: 20444980 Free PMC article.
References
-
- Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–5958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

