Measuring and improving respiratory outcomes in cystic fibrosis lung disease: opportunities and challenges to therapy
- PMID: 19833563
- PMCID: PMC2830746
- DOI: 10.1016/j.jcf.2009.09.003
Measuring and improving respiratory outcomes in cystic fibrosis lung disease: opportunities and challenges to therapy
Abstract
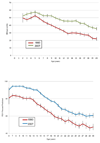
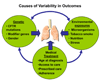
Cystic fibrosis (CF) is a life-shortening disease with significant morbidity. Despite overall improvements in survival, patients with CF experience frequent pulmonary exacerbations and declining lung function, which often accelerates during adolescence. New treatments target steps in the pathogenesis of lung disease, such as the basic defect in CF (CF Transmembrane Conductance Regulator [CFTR]), pulmonary infections, inflammation, and mucociliary clearance. These treatments offer hope but also present challenges to patients, clinicians, and researchers. Comprehensive assessment of efficacy is critical to identify potentially beneficial treatments. Lung function and pulmonary exacerbation are the most commonly used outcome measures in CF clinical research. Other outcome measures under investigation include measures of CFTR function; biomarkers of infection, inflammation, lung injury and repair; and patient-reported outcomes. Molecular diagnostics may help elucidate the complex CF airway microbiome. As new treatments are developed for patients with CF, efforts should be made to balance treatment burden with quality of life. This review highlights emerging treatments, obstacles to optimizing outcomes, and key future directions for research.
Conflict of interest statement
Edith Zemanick has nothing to disclose relevant to this manuscript.
J. Kirk Harris has nothing to disclose relevant to this manuscript.
Steven Conway is a member of the advisory board for Phillips Medizinsysteme Böblingen (Germany) and has recently served on an advisory board for Gilead Sciences, Inc. He has previously served on advisory boards for Roche and Novartis Pharmaceuticals.
Michael W. Konstan has served as a paid consultant to Axcan Pharma, Digestive Care Inc, Genentech, Gilead Sciences, Inc., Novartis Pharmaceuticals, PTC Therpaeutics, Solvay, Transave, Inc., and Vertex Pharmaceuticals, Inc.
Bruce Marshall has nothing to disclose relevant to this manuscript.
Alexandra L. Quittner has received consulting income from Gilead Sciences, Inc., Novartis Pharmaceuticals, Vertex Pharmaceuticals, Inc., and Transave, Inc. She serves on the North American Advisory Group for Genentech (NASAG) and has an investigator-initiated grant from Novartis Pharmaceuticals.
George Retsch-Bogart received support as a site principal investigator to conduct clinical trials in the Cystic Fibrosis Foundation Therapeutics Development Network from Gilead Sciences, Inc. and Inspire Pharmaceuticals.
Lisa Saiman has served on advisory boards for Aridis Pharmaceuticals, Gilead Sciences, Inc., Novartis Pharmaceuticals, and Transave, Inc. She has received research funding from Bayer and Chiesi Pharmaceuticals and has served on the Cystic Fibrosis Foundations Data Safety Monitoring Board.
Frank Accurso has no personal conflict of interest. Dr. Accurso’s institution received funding for clinical trials for agents mentioned in this manuscript from Gilead Sciences, Inc., Vertex Pharmaceuticals, Inspire Pharmaceuticals and PTC Therapeutics.
Figures
References
-
- World Health Organization and Cystic Fibrosis Worldwide. The molecular genetic epidemiology of cystic fibrosis. 2002 Report of a joint meeting of WHO/ECFTN/ICF(M)A/ ECFS.
-
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. - PubMed
-
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1229–1256. - PubMed
-
- Puchelle E, Bajolet O, Abely M. Airway mucus in cystic fibrosis. Paediatr Respir Rev. 2002;3(2):115–119. - PubMed
-
- Quinton PM. Too much salt, too little soda: cystic fibrosis. Sheng Li Xue Bao. 2007;59(4):397–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

