Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia
- PMID: 19833633
- PMCID: PMC2817024
- DOI: 10.3324/haematol.2009.012781
Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia
Abstract
Background: Second-generation tyrosine kinase inhibitors induce cytogenetic responses in approximately 50% of patients with chronic myeloid leukemia in chronic phase in whom imatinib treatment has failed. However, it has not yet been established which of the patients in whom imatinib treatment fails are likely to benefit from therapy with second-generation tyrosine kinase inhibitors.
Design and methods: We analyzed a cohort of 80 patients with chronic myeloid leukemia who were resistant to imatinib and who were treated with dasatinib or nilotinib while still in first chronic phase. We devised a scoring system to predict the probability of these patients achieving complete cytogenetic response when treated with second-generation tyrosine kinase inhibitors.
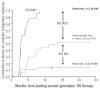
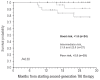
Results: The system was based on three factors: cytogenetic response to imatinib, Sokal score and recurrent neutropenia during imatinib treatment. We validated the score in an independent group of 28 Scottish patients. We also studied the relationship between cytogenetic responses at 3, 6 and 12 months and subsequent outcome. We classified the 80 patients into three categories, those with good risk (n=24), intermediate risk (n=27) and poor risk (n=29) with 2.5-year cumulative incidences of complete cytogenetic response of 100%, 52.2% and 13.8%, respectively (P<0.0001). Moreover, patients who had less than 95% Philadelphia chromosome-positive metaphases at 3 months, those with 35% or less Philadelphia chromosome-positive metaphases at 6 months and patients in complete cytogenetic response at 12 months all had significantly better outcomes than patients with lesser degrees of cytogenetic response.
Conclusions: Factors measurable before starting treatment can accurately predict response to second-generation tyrosine kinase inhibitors. Cytogenetic responses at 3, 6 and 12 months may influence the decision to continue treatment with second-generation tyrosine kinase inhibitors.
Figures



References
-
- Druker B, Guilhot F, O’Brien S, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17. - PubMed
-
- de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358–63. - PubMed
-
- Apperley JF. Part II: management of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8(12):1116–28. - PubMed
-
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–41. - PubMed
-
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

