Rab39a binds caspase-1 and is required for caspase-1-dependent interleukin-1beta secretion
- PMID: 19833722
- PMCID: PMC2787314
- DOI: 10.1074/jbc.M109.046102
Rab39a binds caspase-1 and is required for caspase-1-dependent interleukin-1beta secretion
Erratum in
-
RAB39a binds caspase-1 and is required for caspase-1-dependent interleukin-1β secretion.J Biol Chem. 2016 Nov 18;291(47):24800. doi: 10.1074/jbc.A109.046102. J Biol Chem. 2016. PMID: 27864526 Free PMC article. No abstract available.
Abstract
Interleukin-1beta (IL-1beta) is an important pro-inflammatory cytokine that is secreted by unconventional means in a caspase-1-dependent manner. Using a one-step immunoprecipitation approach to isolate endogenous caspase-1 from the monocytic THP1 cell line, we identified previously undescribed binding partners using mass spectrometry. One of the proteins identified was Rab39a, a member of the Rab GTPase family, a group of proteins that have important roles in protein trafficking and secretion. We confirmed by co-immunoprecipitation that Rab39a binds caspase-1. Knock down of Rab39a with small interfering RNA resulted in diminished levels of secreted IL-1beta but had no effect on induction of pro-IL-1beta mRNA by lipopolysaccharide. Rab39a contains a highly conserved caspase-1 cleavage site and was cleaved in the presence of recombinant caspase-1 or lipopolysaccharide. Finally, overexpression of Rab39a results in an increase in IL-1beta secretion, and furthermore, overexpression of a Rab39a construct lacking the caspase-1 cleavage site leads to an additional increase in IL-1beta secretion. Altogether, our findings show that Rab39a interacts with caspase-1 and suggest that Rab39a functions as a trafficking adaptor linking caspase-1 to IL-1beta secretion.
Figures
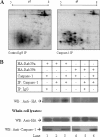
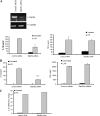


References
-
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J., Elliston K., Ayala J., Casano F., Chin J., Ding G., Egger L., Gaffney E., Limjuco W., Palyha O., Raju S., Rolando A., Salley J., Yamin T., Lee T., Shively J., MacCross M., Mumford R., Schmidt J., Tocci M. (1992) Nature 356, 768–774 - PubMed
-
- Eder C. (2009) Immunobiology 214, 543–553 - PubMed
-
- MacKenzie A., Wilson H. L., Kiss-Toth E., Dower S. K., North R. A., Surprenant A. (2001) Immunity 15, 825–835 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

